Regulation of hepatocyte nuclear factor 1 activity by wild-type and mutant hepatitis B virus X proteins
- PMID: 12021320
- PMCID: PMC136206
- DOI: 10.1128/jvi.76.12.5875-5881.2002
Regulation of hepatocyte nuclear factor 1 activity by wild-type and mutant hepatitis B virus X proteins
Abstract
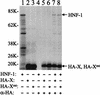
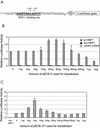
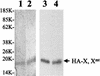
The hepatitis B virus (HBV) core promoter regulates the transcription of two related RNA products named precore RNA and core RNA. Previous studies indicate that a double-nucleotide mutation that occurs frequently during chronic HBV infection converts a nuclear receptor binding site in the core promoter to the binding site of the transcription factor hepatocyte nuclear factor-1 (HNF-1) and specifically suppresses the transcription of the precore RNA. This mutation also changes two codons in the overlapping X protein coding sequence. In this report, we demonstrate that the X protein and its mutant X(mt) can physically bind to HNF-1 both in vitro and in vivo. Further analyses indicate that both X and X(mt) can enhance the gene transactivation and the DNA binding activities of HNF-1. This finding demonstrates for the first time that the X protein can stimulate the DNA binding activity of a homeodomain transcription factor. Interestingly, while both X and X(mt) can stimulate the HNF-1 activities, they differ in their effects: a smaller amount of X(mt) is needed to generate greater transactivation and DNA binding activities of HNF-1. This functional difference between X and X(mt) may have important implications in HBV pathogenesis and is apparently why they have different effects on the core promoter bearing the HNF-1 binding site.
Figures






References
-
- Andrisani, O. M., and S. Barnabas. 1999. The transcriptional function of the hepatitis B virus X protein and its role in hepatocarcinogenesis. Int. J. Oncol. 15:373-379. - PubMed
-
- Barnabas, S., T. Hai, and O. M. Andrisani. 1997. The hepatitis B virus X protein enhances the DNA binding potential and transcription efficacy of bZip transcription factors. J. Biol. Chem. 272:20684-20690. - PubMed
-
- Baumhueter, S., D. B. Mendel, P. B. Conley, C. J. Kuo, C. Turk, M. K. Graves, C. A. Edwards, G. Courtois, and G. R. Crabtree. 1990. HNF-1 shares three sequence motifs with the POU domain proteins and is identical to LF-B1 and APF. Genes Dev. 4:372-379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

