Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4(+) T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication
- PMID: 12021325
- PMCID: PMC136191
- DOI: 10.1128/jvi.76.12.5925-5936.2002
Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4(+) T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication
Abstract
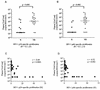
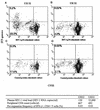
One hallmark of uncontrolled, chronic human immunodeficiency virus type 1 (HIV-1) infection is the absence of strong HIV-1-specific, CD4(+) T-cell-proliferative responses, yet the mechanism underlying this T helper (Th)-cell defect remains controversial. To better understand the impact of HIV-1 replication on Th-cell function, we compared the frequency of CD4(+) Th-cell responses based on production of gamma interferon to lymphoproliferative responses directed against HIV-1 proteins in HIV-1-infected subjects with active in vivo viral replication versus those on suppressed highly active antiretroviral therapy (HAART). No statistically significant differences in the frequencies of cytokine-secreting, HIV-1-specific CD4(+) T cells between the donor groups were found, despite differences in viral load and treatment status. However, HIV-1-specific lymphoproliferative responses were significantly greater in the subjects with HAART suppression than in subjects with active viral replication. Similar levels of HIV-1 RNA were measured in T-cell cultures stimulated with HIV-1 antigens regardless of donor in vivo viral loads, but only HIV-1-specific CD4(+) T cells from subjects with HAART suppression proliferated in vitro, suggesting that HIV-1 replication in vitro does not preclude HIV-1-specific lymphoproliferation. This study demonstrates a discordance between the frequency and proliferative capacity of HIV-1-specific CD4(+) T cells in subjects with ongoing in vivo viral replication and suggests that in vivo HIV-1 replication contributes to the observed defect in HIV-1-specific CD4(+) T-cell proliferation.
Figures



References
-
- Al-Harthi, L., J. Siegel, J. Spritzler, J. Pottage, M. Agnoli, and A. Landay. 2000. Maximum suppression of HIV replication leads to the restoration of HIV-specific responses in early HIV disease. AIDS 14:761-770. - PubMed
-
- Angel, J. B., A. Kumar, K. Parato, L. G. Filion, F. Diaz-Mitoma, P. Daftarian, B. Pham, E. Sun, J. M. Leonard, and D. W. Cameron. 1998. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus saquinavir. J. Infect. Dis. 177:898-904. - PubMed
-
- Angel, J. B., K. G. Parato, A. Kumar, S. Kravcik, A. D. Badley, C. Fex, D. Ashby, E. Sun, and D. W. Cameron. 2001. Progressive human immunodeficiency virus-specific immune recovery with prolonged viral suppression. J. Infect. Dis. 183:546-554. - PubMed
-
- Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112-116. - PubMed
-
- Berzofsky, J. A., A. Bensussan, K. B. Cease, J. F. Bourge, R. Cheynier, Z. Lurhuma, J. J. Salaun, R. C. Gallo, G. M. Shearer, and D. Zagury. 1988. Antigenic peptides recognized by T lymphocytes from AIDS viral envelope-immune humans. Nature 334:706-708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

