Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells
- PMID: 12021326
- PMCID: PMC136219
- DOI: 10.1128/jvi.76.12.5937-5948.2002
Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells
Abstract
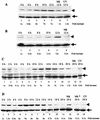
Analyses of mitogen-activated protein kinases (MAPKs) in a mouse hepatitis virus (MHV)-infected macrophage-derived J774.1 cell line showed activation of two MAPKs, p38 MAPK and c-Jun N-terminal kinase (JNK), but not of extracellular signal-regulated kinase (ERK). Activation of MAPKs was evident by 6 h postinfection. However, UV-irradiated MHV failed to activate MAPKs, which demonstrated that MHV replication was necessary for their activation. Several other MHV-permissive cell lines also showed activation of both p38 MAPK and JNK, which indicated that the MHV-induced stress-kinase activation was not restricted to any particular cell type. The upstream kinase responsible for activating MHV-induced p38 MAPK was the MAPK kinase 3. Experiments with a specific inhibitor of p38 MAPK, SB 203580, demonstrated that MHV-induced p38 MAPK activation resulted in the accumulation of interleukin-6 (IL-6) mRNAs and an increase in the production of IL-6, regardless of MHV-induced general host protein synthesis inhibition. Furthermore, MHV production was suppressed in SB 203580-treated cells, demonstrating that activated p38 MAPK played a role in MHV replication. The reduced MHV production in SB 203580-treated cells was, at least in part, due to a decrease in virus-specific protein synthesis and virus-specific mRNA accumulation. Interestingly, there was a transient increase in the amount of phosphorylation of the translation initiation factor 4E (eIF4E) in infected cells, and this eIF4E phosphorylation was p38 MAPK dependent; it is known that phosphorylated eIF4E enhances translation rates of cap-containing mRNAs. Furthermore, the upstream kinase responsible for eIF4E phosphorylation, MAPK-interacting kinase 1, was also phosphorylated and activated in response to MHV infection. Our data suggested that host cells, in response to MHV replication, activated p38 MAPK, which subsequently phosphorylated eIF4E to efficiently translate certain host proteins, including IL-6, during virus-induced severe host protein synthesis inhibition. MHV utilized this p38 MAPK-dependent increase in eIF4E phosphorylation to promote virus-specific protein synthesis and subsequent progeny virus production. Enhancement of virus-specific protein synthesis through virus-induced eIF4E activation has not been reported in any other viruses.
Figures

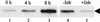

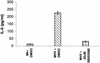
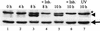


References
-
- Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

