Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex
- PMID: 12021330
- PMCID: PMC136238
- DOI: 10.1128/jvi.76.12.5974-5984.2002
Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex
Abstract
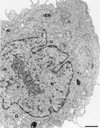
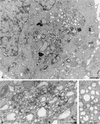
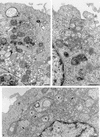
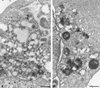
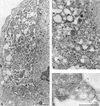
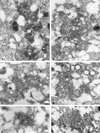
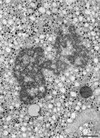
Plus-strand RNA viruses characteristically replicate their genome in association with altered cellular membranes. In the present study, the capacity of hepatitis C virus (HCV) proteins to elicit intracellular membrane alterations was investigated by expressing, in tetracycline-regulated cell lines, a comprehensive panel of HCV proteins individually as well as in the context of the entire HCV polyprotein. As visualized by electron microscopy (EM), expression of the combined structural proteins core-E1-E2-p7, the NS3-4A complex, and protein NS4B induced distinct membrane alterations. By immunogold EM (IEM), the membrane-altering proteins were always found to localize to the respective altered membranes. NS4B, a protein of hitherto unknown function, induced a tight structure, designated membranous web, consisting of vesicles in a membranous matrix. Expression of the entire HCV polyprotein gave rise to membrane budding into rough endoplasmic reticulum vacuoles, to the membranous web, and to tightly associated vesicles often surrounding the membranous web. By IEM, all HCV proteins were found to be associated with the NS4B-induced membranous web, forming a membrane-associated multiprotein complex. A similar web-like structure in livers of HCV-infected chimpanzees was previously described (Pfeifer et al., Virchows Arch. B., 33:233-243, 1980). In view of this finding and the observation that all HCV proteins accumulate on the membranous web, we propose that the membranous web forms the viral replication complex in HCV-infected cells.
Figures

References
-
- Aldabe, R., and L. Carrasco. 1995. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem. Biophys. Res. Commun. 206:64-76. - PubMed
-
- Barba, G., F. Harper, T. Harada, M. Kohara, S. Goulinet, Z. Matsuura, G. Eder, Z. Schaff, M. J. Chapman, T. Miyamura, and C. Bréchot. 1997. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc. Natl. Acad. Sci. USA 94:1200-1205. - PMC - PubMed
-
- Bartenschlager, R., and V. Lohmann. 2000. Replication of hepatitis C virus. J. Gen. Virol. 81:1631-1648. - PubMed
-
- Bienz, K., and D. Egger. 1995. Immunocytochemistry and in situ hybridization in the electron microscope: combined application in the study of virus-infected cells. Histochem. Cell Biol. 103:325-338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

