Virulence and reduced fitness of simian immunodeficiency virus with the M184V mutation in reverse transcriptase
- PMID: 12021341
- PMCID: PMC136201
- DOI: 10.1128/jvi.76.12.6083-6092.2002
Virulence and reduced fitness of simian immunodeficiency virus with the M184V mutation in reverse transcriptase
Abstract
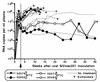
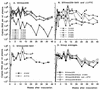
Drug-resistant mutants with a methionine-to-valine substitution at position 184 of reverse transcriptase (M184V) emerged within 5 weeks of initiation of therapy in four newborn macaques infected with simian immunodeficiency virus (SIVmac251) and treated with lamivudine (3TC) or emtricitabine [(-)-FTC] (two animals per drug). Thus, this animal model mimics the rapid emergence of M184V mutants of HIV-1 during 3TC therapy of human patients. One animal of each treatment group developed fatal immunodeficiency at 12 weeks of age, which is similar to the rapid disease course seen in most untreated SIVmac251-infected infant macaques. To further evaluate the effect of the M184V mutation on viral fitness and virulence, groups of juvenile macaques were inoculated with the molecular clone SIVmac239 with either the wild-type sequence (group A [n = 5]) or the M184V sequence (SIVmac239-184V; group B [n = 5] and group C [n = 2]). The two SIVmac239-184V-infected animals of group C did not receive any drug treatment, and in both animals the virus population reverted to predominantly wild type (184M) by 8 weeks after inoculation. The other five SIVmac239-184V-infected animals (group B) were treated with (-)-FTC to prevent reversion. Although virus levels 1 week after inoculation were lower in the SIVmac239-184V-infected macaques than in the SIVmac239-infected animals, no significant differences were observed from week 2 onwards. Two animals in each group developed AIDS and were euthanized, while all other animals were clinically stable at 46 weeks of infection. These data demonstrate that the M184V mutation in SIV conferred a slightly reduced fitness but did not affect disease outcome.
Figures
References
-
- Back, N. K., M. Nijhuis, W. Keulen, C. A. B. Boucher, B. O. Oude Essink, A. B. van Kuilenberg, A. H. van Gennip, and B. Berkhout. 1996. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 15:4040-4049. - PMC - PubMed
-
- Balzarini, J., M. Weeger, M.-J. Camarasa, E. De Clercq, and K. Überla. 1995. Sensitivity/resistance profile of a simian immunodeficiency virus containing the reverse transcriptase gene of human immunodeficiency virus type 1 (HIV-1) toward the HIV-1 specific non-nucleoside reverse transcriptase inhibitors. Biochem. Biophys. Res. Commun. 211:850-856. - PubMed
-
- Catucci, M., G. Venturi, L. Romano, M. L. Riccio, A. De Milito, P. E. Valensin, and M. Zazzi. 1999. Development and significance of the HIV-1 reverse transcriptase M184V mutation during combination therapy with lamivudine, zidovudine, and protease inhibitors. J. Acquir. Immune Defic. Syndr. 21:203-208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

