Half-life of the duck hepatitis B virus covalently closed circular DNA pool in vivo following inhibition of viral replication
- PMID: 12021368
- PMCID: PMC136192
- DOI: 10.1128/jvi.76.12.6356-6363.2002
Half-life of the duck hepatitis B virus covalently closed circular DNA pool in vivo following inhibition of viral replication
Abstract
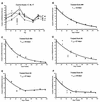
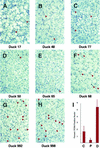
Covalently closed circular DNA (cccDNA) is a crucial intermediate in the replication of hepadnaviruses. We inhibited the replication of duck hepatitis B virus in congenitally infected ducks with a combination of lamivudine and a dideoxyguanosine prodrug. Inhibition of viral replication should prevent renewal of the cccDNA pool, and its decay was measured in liver biopsy samples collected over a 5-month period. In three ducks, the cccDNA pools declined exponentially, with half-lives ranging from 35 to 57 days. In two others, the pools declined exponentially for about 70 days but then stabilized at about 6 copies/diploid genome. The selection of drug-resistant virus mutants is an unlikely explanation for this unexpected stabilization of cccDNA levels. Liver sections stained for the cell division marker PCNA showed that animals in which cccDNA loss was continuous had significantly greater numbers of PCNA-positive nuclei than did those animals in which cccDNA levels had plateaued.
Figures


References
-
- Addison, W. R., W. W. S. Wong, K. P. Fischer, and D. L. J. Tyrrell. 2000. A quantitative competitive PCR assay for the covalently closed circular form of the duck hepatitis B virus. Antivir. Res. 48:27-37. - PubMed
-
- Chyama, K., Y. Suzuki, M. Kobayashi, M. Kobayashi, A. Tsubota, M. Hashimoto, Y. Miyano, H. Koike, M. Kobayashi, I. Koida, Y. Arase, S. Saitoh, N. Murashima, K. Ikeda, and H. Kumada. 1998. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology 27:1711-1716. - PubMed
-
- Civitico, G. M., and S. A. Locarnini. 1994. The half-life of duck hepatitis B virus supercoiled DNA in congenitally infected primary hepatocyte cultures. Virology 203:81-89. - PubMed
-
- Dandri, M., M. R. Burda, H. Will, and J. Petersen. 2000. Increased hepatocyte turnover and inhibition of woodchuck hepatitis B virus replication by adefovir in vitro do not lead to reduction of the closed circular DNA. Hepatology 32:139-146. - PubMed
-
- Dienstag, J. L., R. P. Perrillo, E. R. Schiff, M. Bartholomew, C. Vicary, and M. Rubin. 1995. A preliminary trial of lamivudine for chronic hepatitis B infection. N. Engl. J. Med. 333:1657-1661. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

