Conformational changes in chemically modified Escherichia coli thioredoxin monitored by H/D exchange and electrospray ionization mass spectrometry
- PMID: 12021431
- PMCID: PMC2373629
- DOI: 10.1110/ps.3140102
Conformational changes in chemically modified Escherichia coli thioredoxin monitored by H/D exchange and electrospray ionization mass spectrometry
Abstract
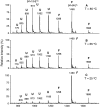
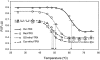
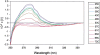
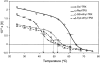
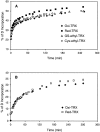
Hydrogen/deuterium (H/D) exchange in combination with electrospray ionization mass spectrometry and near-ultraviolet (UV) circular dichroism (CD) was used to study the conformational properties and thermal unfolding of Escherichia coli thioredoxin and its Cys32-alkylated derivatives in 1% acetic acid (pH 2.7). Thermal unfolding of oxidized (Oxi) and reduced (Red) -thioredoxin (TRX) and Cys-32-ethylglutathionyl (GS-ethyl-TRX) and Cys-32-ethylcysteinyl (Cys-ethyl-TRX), which are derivatives of Red-TRX, follow apparent EX1 kinetics as charge-state envelopes, H/D mass spectral exchange profiles, and near-UV CD appear to support a two-state folding/unfolding model. Minor mass peaks in the H/D exchange profiles and nonsuperimposable MS- and CD-derived melting curves, however, suggest the participation of unfolding intermediates leading to the conclusion that the two-state model is an oversimplification of the process. The relative stabilities as measured by melting temperatures by both CD and mass spectral charge states are, Oxi-TRX, GS-ethyl-TRX, Cys-ethyl-TRX, and Red-TRX. The introduction of the Cys-32-ethylglutathionyl group provides extra stabilization that results from additional hydrogen bonding interactions between the ethylglutathionyl group and the protein. Near-UV CD data show that the local environment near the active site is perturbed to almost an identical degree regardless of whether alkylation at Cys-32 is by the ethylglutathionyl group, or the smaller, nonhydrogen-bonding ethylcysteinyl group. Mass spectral data, however, indicate a tighter structure for GS-ethyl-TRX.
Figures
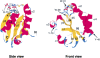


Similar articles
-
Intramolecular interactions in chemically modified Escherichia coli thioredoxin monitored by hydrogen/deuterium exchange and electrospray ionization mass spectrometry.Biochemistry. 2001 Dec 4;40(48):14413-21. doi: 10.1021/bi0115941. Biochemistry. 2001. PMID: 11724553
-
Thermal denaturation of Escherichia coli thioredoxin studied by hydrogen/deuterium exchange and electrospray ionization mass spectrometry: monitoring a two-state protein unfolding transition.Biochemistry. 1999 Jan 19;38(3):1136-43. doi: 10.1021/bi981938w. Biochemistry. 1999. PMID: 9894011
-
Effects of buried charged groups on cysteine thiol ionization and reactivity in Escherichia coli thioredoxin: structural and functional characterization of mutants of Asp 26 and Lys 57.Biochemistry. 1997 Mar 4;36(9):2622-36. doi: 10.1021/bi961801a. Biochemistry. 1997. PMID: 9054569
-
Mass spectrometric approaches using electrospray ionization charge states and hydrogen-deuterium exchange for determining protein structures and their conformational changes.Mol Cell Proteomics. 2004 Jan;3(1):10-23. doi: 10.1074/mcp.R300010-MCP200. Epub 2003 Nov 16. Mol Cell Proteomics. 2004. PMID: 14623985 Review.
-
Thioredoxin from Escherichia coli as a Role Model of Molecular Recognition, Folding, Dynamics and Function.Protein Pept Lett. 2015;22(9):801-15. doi: 10.2174/0929866522666150707114309. Protein Pept Lett. 2015. PMID: 26149400 Review.
Cited by
-
Scope and utility of hydrogen exchange as a tool for mapping landscapes.Protein Sci. 2007 Nov;16(11):2378-90. doi: 10.1110/ps.072994207. Protein Sci. 2007. PMID: 17962401 Free PMC article.
-
Hydrogen atom scrambling in selectively labeled anionic peptides upon collisional activation by MALDI tandem time-of-flight mass spectrometry.J Am Soc Mass Spectrom. 2008 Dec;19(12):1719-25. doi: 10.1016/j.jasms.2008.05.021. Epub 2008 Jun 11. J Am Soc Mass Spectrom. 2008. PMID: 18640053
-
Optimization of Feasibility Stage for Hydrogen/Deuterium Exchange Mass Spectrometry.J Am Soc Mass Spectrom. 2018 Mar;29(3):623-629. doi: 10.1007/s13361-017-1860-3. Epub 2018 Jan 3. J Am Soc Mass Spectrom. 2018. PMID: 29299838
-
Rapid refinement of crystallographic protein construct definition employing enhanced hydrogen/deuterium exchange MS.Proc Natl Acad Sci U S A. 2004 Jan 20;101(3):751-6. doi: 10.1073/pnas.0307204101. Epub 2004 Jan 8. Proc Natl Acad Sci U S A. 2004. PMID: 14715906 Free PMC article.
-
Conformational analysis of HAMLET, the folding variant of human alpha-lactalbumin associated with apoptosis.Protein Sci. 2004 May;13(5):1322-30. doi: 10.1110/ps.03474704. Epub 2004 Apr 9. Protein Sci. 2004. PMID: 15075403 Free PMC article.
References
-
- Adler, S. and Modrich, P. 1983. T7-induced DNA polymerase. Requirement for thioredoxin sulfhydryl groups. J. Biol. Chem. 258 6956–6962. - PubMed
-
- Arrington, C.B. and Robertson, A.D. 2000. Correlated motions in native proteins from MS analysis of NH exchange: Evidence for a manifold of unfolding reactions in ovomucoid third domain. J. Mol. Biol. 300 221–232. - PubMed
-
- Englander, S.W. and Kallenbach N.R. 1984. Hydrogen exchange and structural dynamics of proteins and nucleic acids. Quart. Rev. Biophys. 16 521–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

