Product-conformation-driven ligation of peptides by V8 protease
- PMID: 12021437
- PMCID: PMC2373616
- DOI: 10.1110/ps.0201302
Product-conformation-driven ligation of peptides by V8 protease
Abstract
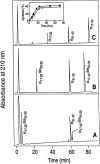
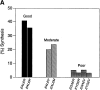
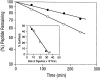
Organic co-solvent-induced secondary conformation of alpha(17-40) of human hemoglobin facilitates the splicing of E30-R31 in a mixture of its complementary segments by V8 protease. The amino acid sequence of alpha(17-40) has been conceptualized by the general structure FR(I)-EALER-FR(II) and the pentapeptide sequence EALER playing a major role in inducing the alpha-helical conformation. The primary structure of alpha(17-40) has been engineered in multiple ways to perturb one, two, or all three regions and the influence of the organic co-solvent-induced conformation and the concomitant resistance of E30-R31 peptide bond to V8 protease digestion has been investigated. The central pentapeptide (EALER), referred to here as splicedon,(3) appears to dictate a primary role in facilitating the splicing reaction. When the same flanking regions are used, (1) splicedons that carry amino acid residues of low alpha-helical potential, for example G at position 2 or 3 of the splicedon, generate a conformational trap of very low thermodynamic stability, giving an equilibrium yield of only 3%-5%; (2) splicedons with amino acid residues of good alpha-helical potential generate a conformational trap of medium thermodynamic stability and give an equilibrium yield of 20%-25%; (3) the splicedons with amino residues of good alpha-helical potential and also an amino acid that can generate an i, i + 4 side-chain carboxylate-guanidino (amino) interaction, a conformational trap of maximum thermodynamic stability is generated, giving an equilibrium yield of 45%-50%; and (4) the thermodynamic stability of the conformational trap of the spliced peptide is also influenced by the amino acid composition of the flanking regions. The V8 protease resistance of the spliced peptide bond is not a direct correlate of the amount of alpha-helical conformation induced into the product. The results of this study reflect the unique role of the splicedon in translating the organic co-solvent-induced product conformation as a site-specific stabilization of the spliced peptide bond. It is speculated that the splicedon with higher alpha-helical potential as compared to either one of the flanking regions achieves this by integrating its potential with that of the flanking region(s). Exchange of flanking regions with the products of other V8 protease-catalyzed splicing reactions will help to establish the general primary structural requirements of this class of splicing reactions and facilitate their application in modular construction of proteins.
Figures


Similar articles
-
Product conformation driven splicing of unprotected peptides by reverse proteolysis: influence of intrinsic and extrinsic factors.Indian J Biochem Biophys. 2002 Aug;39(4):240-52. Indian J Biochem Biophys. 2002. PMID: 22908414
-
Chemistry of the "molecular trap" of protease-catalyzed splicing reaction of complementary segments of alpha-subunit of hemoglobin A.J Protein Chem. 1998 Oct;17(7):669-78. doi: 10.1007/BF02780969. J Protein Chem. 1998. PMID: 9853682
-
Conformationally driven protease-catalyzed splicing of peptide segments: V8 protease-mediated synthesis of fragments derived from thermolysin and ribonuclease A.Protein Sci. 1997 Oct;6(10):2233-41. doi: 10.1002/pro.5560061018. Protein Sci. 1997. PMID: 9336846 Free PMC article.
-
An enigmatic peptide ligation reaction: protease-catalyzed oligomerization of a native protein segment in neat aqueous solution.Protein Sci. 2000 Apr;9(4):734-41. doi: 10.1110/ps.9.4.734. Protein Sci. 2000. PMID: 10794415 Free PMC article.
-
[A turning point in the knowledge of the structure-function-activity relations of elastin].J Soc Biol. 2001;195(2):181-93. J Soc Biol. 2001. PMID: 11727705 Review. French.
Cited by
-
Hemoglobin Einstein: semisynthetic deletion in the B-helix of the alpha-chain.Protein Sci. 2004 May;13(5):1266-75. doi: 10.1110/ps.03567804. Protein Sci. 2004. PMID: 15096632 Free PMC article.
References
-
- Acharya, A.S., Sahni, G., Roy, R.P., and Manjula, B.N. 1993. Quality of the helix induced into enzymatically ligated peptides dictates the splicing of non-interacting peptides: A comparative study of the V8 protease catalyzed splicing of complementary segments of RNase-S peptide and α-globin. Ind. J. Chem. 32B 1–10.
-
- Cerovsky, V., Wunsch, E., and Brass, J. 1997. Enzymatic semisynthesis of dicarba analogs of calcitonin. Eur. J. Biochem. 247 231–237. - PubMed
-
- Chaiken, I.M., Ando, S., and Fessina, G. 1987. Enzymatic synthesis and formulation of biologically active peptides. Adv. Biosci. 65 141–150.
-
- Chakrabartty, A. and Baldwin, R.L. 1995. Stability of α-helices. Adv. Prot. Chem. 46 141–176. - PubMed
-
- Chakrabartty, A., Schellman, J.A., and Baldwin, R.L. 1991. Large differences in the helix propensities of alanine and glycine. Nature 351 586–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

