TEM-1 beta-lactamase as a scaffold for protein recognition and assay
- PMID: 12021449
- PMCID: PMC2373628
- DOI: 10.1110/ps.0203102
TEM-1 beta-lactamase as a scaffold for protein recognition and assay
Abstract
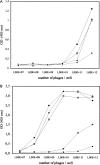
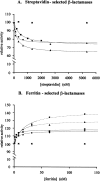
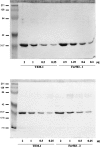
A large number of different proteins or protein domains have been investigated as possible scaffolds to engineer antibody-like molecules. We have previously shown that the TEM-1 beta-lactamase can accommodate insertions of random sequences in two loops surrounding its active site without compromising its activity. From the libraries that were generated, active enzymes binding with high affinities to monoclonal antibodies raised against prostate-specific antigen, a protein unrelated to beta-lactamase, could be isolated. Antibody binding was shown to affect markedly the enzyme activity. As a consequence, these enzymes have the potential to be used as signaling molecules in direct or competitive homogeneous immunoassay. Preliminary results showed that beta-lactamase clones binding to streptavidin could also be isolated, indicating that some enzymes in the libraries have the ability to recognize proteins other than antibodies. In this paper, we show that, in addition to beta-lactamases binding to streptavidin, beta-lactamase clones binding to horse spleen ferritin and beta-galactosidase could be isolated. Affinity maturation of a clone binding to ferritin allowed obtaining beta-lactamases with affinities comprised between 10 and 20 nM (Kd) for the protein. Contrary to what was observed for beta-lactamases issued from selections on antibodies, enzyme complexation induced only a modest effect on enzyme activity, in the three cases studied. This kind of enzyme could prove useful in replacement of enzyme-conjugated antibodies in enzyme-linked immunosorbant assays (ELISA) or in other applications that use antibodies conjugated to an enzyme.
Figures



References
-
- Benito, A., Feliu, J.X., and Villaverde, A. 1996. β-Galactosidase enzymatic activity as a molecular probe to detect specific antibodies. J. Biol. Chem. 271 21251–21256. - PubMed
-
- Brennan, C., Christianson, K., Surowy, T., and Mandecki, W. 1994. Modulation of enzyme activity by antibody binding to an alkaline phosphatase-epitope hybrid protein. Protein Eng. 7 509–514. - PubMed
-
- Cadwell, R.C. and Joyce, G.F. 1994. Mutagenic PCR. PCR Methods Applic. 3 S136–S140. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

