Covalent cross-linking of proteins without chemical reagents
- PMID: 12021454
- PMCID: PMC2373635
- DOI: 10.1110/ps.4390102
Covalent cross-linking of proteins without chemical reagents
Abstract
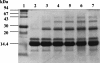
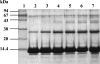
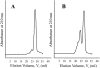
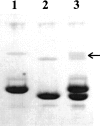
A facile method for the formation of zero-length covalent cross-links between protein molecules in the lyophilized state without the use of chemical reagents has been developed. The cross-linking process is performed by simply sealing lyophilized protein under vacuum in a glass vessel and heating at 85 degrees C for 24 h. Under these conditions, approximately one-third of the total protein present becomes cross-linked, and dimer is the major product. Chemical and mass spectroscopic evidence obtained shows that zero-length cross-links are formed as a result of the condensation of interacting ammonium and carboxylate groups to form amide bonds between adjacent molecules. For the protein examined in the most detail, RNase A, the cross-linked dimer has only one amide cross-link and retains the enzymatic activity of the monomer. The in vacuo cross-linking procedure appears to be general in its applicability because five different proteins tested gave substantial cross-linking, and co-lyophilization of lysozyme and RNase A also gave a heterogeneous covalently cross-linked dimer.
Figures




Similar articles
-
Application of zero-length cross-linking to form lysozyme, horseradish peroxidase and lysozyme-peroxidase dimers: Activity and stability.Int J Biol Macromol. 2007 Dec 1;41(5):624-30. doi: 10.1016/j.ijbiomac.2007.08.004. Epub 2007 Aug 26. Int J Biol Macromol. 2007. PMID: 17915308
-
A novel cross-linked RNase A dimer with enhanced enzymatic properties.Proteins. 2007 Jan 1;66(1):183-95. doi: 10.1002/prot.21144. Proteins. 2007. PMID: 17044066
-
Carbodiimide EDC induces cross-links that stabilize RNase A C-dimer against dissociation: EDC adducts can affect protein net charge, conformation, and activity.Bioconjug Chem. 2009 Aug 19;20(8):1459-73. doi: 10.1021/bc9001486. Epub 2009 Jul 16. Bioconjug Chem. 2009. PMID: 19606852
-
Chemical cross-linking and mass spectrometry to map three-dimensional protein structures and protein-protein interactions.Mass Spectrom Rev. 2006 Jul-Aug;25(4):663-82. doi: 10.1002/mas.20082. Mass Spectrom Rev. 2006. PMID: 16477643 Review.
-
Analysis of protein-nucleic acid interactions by photochemical cross-linking and mass spectrometry.Mass Spectrom Rev. 2002 May-Jun;21(3):163-82. doi: 10.1002/mas.10024. Mass Spectrom Rev. 2002. PMID: 12476441 Review.
Cited by
-
Intra- vs Intermolecular Cross-Links in Poly(methyl methacrylate) Networks Containing Enamine Bonds.Macromolecules. 2022 May 10;55(9):3627-3636. doi: 10.1021/acs.macromol.1c02607. Epub 2022 Apr 26. Macromolecules. 2022. PMID: 35578611 Free PMC article.
-
Kinetics and mechanisms of deamidation and covalent amide-linked adduct formation in amorphous lyophiles of a model asparagine-containing Peptide.Pharm Res. 2012 Oct;29(10):2722-37. doi: 10.1007/s11095-011-0591-6. Epub 2011 Oct 18. Pharm Res. 2012. PMID: 22006203
-
Correlations between Photodegradation of Bisretinoid Constituents of Retina and Dicarbonyl Adduct Deposition.J Biol Chem. 2015 Nov 6;290(45):27215-27227. doi: 10.1074/jbc.M115.680363. Epub 2015 Sep 22. J Biol Chem. 2015. PMID: 26400086 Free PMC article.
-
Nanodiamonds as Carriers for Address Delivery of Biologically Active Substances.Nanoscale Res Lett. 2010 Jan 19;5(3):631-636. doi: 10.1007/s11671-010-9526-0. Nanoscale Res Lett. 2010. PMID: 20672079 Free PMC article.
-
"Zero-length" cross-linking in solid state as an approach for analysis of protein-protein interactions.Protein Sci. 2006 Mar;15(3):429-40. doi: 10.1110/ps.051685706. Protein Sci. 2006. PMID: 16501223 Free PMC article.
References
-
- Chang, T.M. 1998. Modified hemoglobin blood substitutes: Present status and future perspectives. Biotechnol. Annu. Rev. 4 75–112. - PubMed
-
- Fancy, D.A. 2000. Elucidation of protein–protein interactions using chemical cross-linking or label transfer techniques. Curr. Opin. Chem. Biol. 4 28–33. - PubMed
-
- Gaur, D., Swaminathan, S., and Batra, J.K. 2001. Interaction of human pancreatic ribonuclease with human ribonuclease inhibitor. Generation of inhibitor-resistant cytotoxic variants. J. Biol. Chem. 276 24978–24984. - PubMed
-
- Jones, R.T., Shih, D.T., Fujita, T.S., Song, Y., Xiao, H., Head, C., and Kluger, R. 1996. A doubly cross-linked human hemoglobin. Effects of cross-links between different subunits. J. Biol. Chem. 271 675–680. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

