Identification of protein tyrosine phosphatase 1B and casein as substrates for 124-v-Mos
- PMID: 12022922
- PMCID: PMC102758
- DOI: 10.1186/1471-2091-3-6
Identification of protein tyrosine phosphatase 1B and casein as substrates for 124-v-Mos
Abstract
Background: The mos proto-oncogene encodes a cytoplasmic serine/threonine-specific protein kinase with crucial function during meiotic cell division in vertebrates. Based on oncogenic amino acid substitutions the viral derivative, 124-v-Mos, displays constitutive protein kinase activity and functions independent of unknown upstream effectors of mos protein kinase. We have utilized this property of 124-v-Mos and screened for novel mos substrates in immunocomplex kinase assays in vitro.
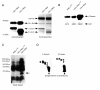
Results: We generated recombinant 124-v-Mos using the baculovirus expression system in Spodoptera frugiperda cells and demonstrated constitutive kinase activity by the ability of 124-v-Mos to auto-phosphorylate and to phosphorylate vimentin, a known substrate of c-Mos. Using this approach we analyzed a panel of acidic and basic substrates in immunocomplex protein kinase assays and identified novel in vitro substrates for 124-v-Mos, the protein tyrosine phosphatase 1B (PTP1B), alpha-casein and beta-casein. We controlled mos-specific phosphorylation of PTP1B and casein in comparative assays using a synthetic kinase-inactive 124-v-Mos mutant and further, tryptic digests of mos-phosphorylated beta-casein identified a phosphopeptide specifically targeted by wild-type 124-v-Mos. Two-dimensional phosphoamino acid analyses showed that 124-v-mos targets serine and threonine residues for phosphorylation in casein at a 1:1 ratio but auto-phosphorylation occurs predominantly on serine residues.
Conclusion: The mos substrates identified in this study represent a basis to approach the identification of the mos-consensus phosphorylation motif, important for the development of specific inhibitors of the Mos protein kinase.
Figures



Similar articles
-
Kinase activities of c-Mos and v-Mos proteins: a single amino acid exchange is responsible for constitutive activation of the 124 v-Mos kinase.Oncogene. 1995 Feb 16;10(4):623-30. Oncogene. 1995. PMID: 7862439
-
Multiple phosphorylation of chicken protein tyrosine phosphatase 1 and human protein tyrosine phosphatase 1B by casein kinase II and p60c-src in vitro.Biochem Biophys Res Commun. 1998 May 8;246(1):238-42. doi: 10.1006/bbrc.1998.8605. Biochem Biophys Res Commun. 1998. PMID: 9600099
-
Evidence of a functional interaction between serine 3 and serine 25 Mos phosphorylation sites. A dominant inhibitory role of serine 25 phosphorylation on Mos protein kinase.J Biol Chem. 1998 Jun 26;273(26):15946-53. doi: 10.1074/jbc.273.26.15946. J Biol Chem. 1998. PMID: 9632642
-
Characterization of MEK1 phosphorylation by the v-Mos protein.Oncogene. 1995 Apr 20;10(8):1683-8. Oncogene. 1995. PMID: 7731726
-
The casein kinase II beta subunit binds to Mos and inhibits Mos activity.Mol Cell Biol. 1997 Apr;17(4):1904-12. doi: 10.1128/MCB.17.4.1904. Mol Cell Biol. 1997. PMID: 9121438 Free PMC article.
References
-
- Maxwell SA, Arlinghaus RB. Serine kinase activity associated with Maloney murine sarcoma virus-124-encoded p37mos. Virology. 1985;143:321–333. - PubMed
-
- Singh B, Wittenberg C, Hannink M, Reed SI, Donoghue DJ, Arlinghaus RB. The histidine-221 to tyrosine substitution in v-mos abolishes its biological function and its protein kinase activity. Virology. 1988;164:114–120. - PubMed
-
- Propst F, Vande Woude GF. Expression of c-mos proto-oncogene transcripts in mouse tissues. Nature. 1985;315:516–518. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

