Oct4 distribution and level in mouse clones: consequences for pluripotency
- PMID: 12023300
- PMCID: PMC186284
- DOI: 10.1101/gad.966002
Oct4 distribution and level in mouse clones: consequences for pluripotency
Abstract
Somatic cell clones often fail at a developmental stage coincident with commencement of differentiation. The transcription factor Oct4 is expressed during cleavage stages and is essential for the differentiation of the blastocyst. Oct4 expression becomes restricted to the inner cell mass and epiblast. After gastrulation Oct4 is active only in germ cells and is silent in somatic cells. Here, Oct4 and an Oct4-GFP transgene were used as markers for which gene reprogramming could be directly related to the developmental potential of somatic cell clones. Cumulus cell clones initiated Oct4 expression at the correct stage but showed an incorrect spatial expression in the majority of blastocysts. The ability of clones to form outgrowths was reduced, and the outgrowths had low or even undetectable levels of Oct4 RNA or GFP. The quality of GFP signals in blastocysts correlated with the ability to generate outgrowths that maintain GFP expression and the frequency of embryonic stem (ES) cell derivation. Abnormal Oct4 expression in clones is either directly or indirectly caused by reprogramming errors and is indicative of a general failure to reset the genetic program. The abnormal Oct4 expression may be associated with aberrant expression of other crucial developmental genes, leading to abnormalities at various embryonic stages. Regardless of other genes, the variations observed in Oct4 levels alone account for the majority of failures currently observed for somatic cell cloning.
Figures
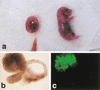

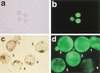

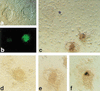

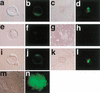
Comment in
-
Cloning v. clowning.Genes Dev. 2002 May 15;16(10):1163-6. doi: 10.1101/gad.998302. Genes Dev. 2002. PMID: 12023296 No abstract available.
References
-
- Abbondanzo SJ, Gadi I, Stewart CL. Derivation of embryonic stem cell lines. Methods Enzymol. 1993;225:803–823. - PubMed
-
- Amano T, Tani T, Kato Y, Tsunoda Y. Mouse cloned from embryonic stem (ES) cells synchronized in metaphase with nocodazole. J Exp Zool. 2001;289:139–145. - PubMed
-
- Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. - PubMed
-
- Cheong HT, Takahashi Y, Kanagawa H. Birth of mice after transplantation of early cell-cycle-stage embryonic nuclei into enucleated oocytes. Biol Reprod. 1993;48:958–963. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
