A Krüppel-associated box-zinc finger protein, NT2, represses cell-type-specific promoter activity of the alpha 2(XI) collagen gene
- PMID: 12024037
- PMCID: PMC133841
- DOI: 10.1128/MCB.22.12.4256-4267.2002
A Krüppel-associated box-zinc finger protein, NT2, represses cell-type-specific promoter activity of the alpha 2(XI) collagen gene
Erratum in
-
A Krüppel-associated box-zinc finger protein, NT2, represses cell-type-specific promoter activity of the alpha2(XI) collage gene.Mol Cell Biol. 2006 Nov;26(21):8215-6. doi: 10.1128/MCB.01723-06. Mol Cell Biol. 2006. Retraction in: Mol Cell Biol. 2009 Jun;29(12):3453. doi: 10.1128/MCB.00411-09. PMID: 17047255 Free PMC article. Retracted. No abstract available.
Retraction in
-
Retraction.Mol Cell Biol. 2009 Jun;29(12):3453. doi: 10.1128/MCB.00411-09. Mol Cell Biol. 2009. PMID: 19474393 Free PMC article. No abstract available.
Abstract
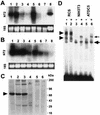
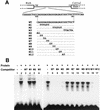
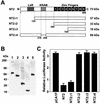
Type XI collagen is composed of three chains, alpha 1(XI), alpha 2(XI), and alpha 3(XI), and plays a critical role in the formation of cartilage collagen fibrils and in skeletal morphogenesis. It was previously reported that the -530-bp promoter segment of the alpha 2(XI) collagen gene (Col11a2) was sufficient for cartilage-specific expression and that a 24-bp sequence from this segment was able to switch promoter activity from neural tissues to cartilage in transgenic mice when this sequence was placed in the heterologous neurofilament light gene (NFL) promoter. To identify a protein factor that bound to the 24-bp sequence of the Col11a2 promoter, we screened a mouse limb bud cDNA expression library in the yeast one-hybrid screening system and obtained the cDNA clone NT2. Sequence analysis revealed that NT2 is a zinc finger protein consisting of a Krüppel-associated box (KRAB) and is a homologue of human FPM315, which was previously isolated by random cloning and sequencing. The KRAB domain has been found in a number of zinc finger proteins and implicated as a transcriptional repression domain, although few target genes for KRAB-containing zinc finger proteins has been identified. Here, we demonstrate that NT2 functions as a negative regulator of Col11a2. In situ hybridization analysis of developing mouse cartilage showed that NT2 mRNA is highly expressed by hypertrophic chondrocytes but is minimally expressed by resting and proliferating chondrocytes, in an inverse correlation with the expression patterns of Col11a2. Gel shift assays showed that NT2 bound a specific sequence within the 24-bp site of the Col11a2 promoter. We found that Col11a2 promoter activity was inhibited by transfection of the NT2 expression vector in RSC cells, a chondrosarcoma cell line. The expression vector for mutant NT2 lacking the KRAB domain failed to inhibit Col11a2 promoter activity. These results demonstrate that KRAB-zinc finger protein NT2 inhibits transcription of its physiological target gene, suggesting a novel regulatory mechanism of cartilage-specific expression of Col11a2.
Figures





Comment in
-
Finding of scientific misconduct.NIH Guide Grants Contracts (Bethesda). 2009 Feb 20:NOT-OD-09-051. NIH Guide Grants Contracts (Bethesda). 2009. PMID: 19238680 Free PMC article. No abstract available.
References
-
- Adamson, M. C., J. Silver, and C. A. Kozak. 1991. The mouse homolog of the Gibbon ape leukemia virus receptor: genetic mapping and a possible receptor function in rodents. Virology 183:778-781. - PubMed
-
- Berg, J. M., and Y. Shi. 1996. The galvanization of biology: a growing appreciation for the roles of zinc. Science 271:1081-1085. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
