Up-regulation of the Yersinia enterocolitica yop regulon by deletion of the flagellum master operon flhDC
- PMID: 12029037
- PMCID: PMC135097
- DOI: 10.1128/JB.184.12.3214-3223.2002
Up-regulation of the Yersinia enterocolitica yop regulon by deletion of the flagellum master operon flhDC
Abstract
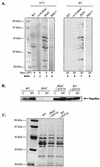
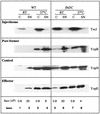
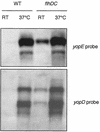
The Yop virulon enables extracellularly located Yersinia, in close contact with a eukaryotic target cell, to inject bacterial toxic proteins directly into the cytosol of this cell. Several Ysc proteins, forming the Yop secretion apparatus, display homology with proteins of the flagellar basal body. To determine whether this relationship could extend to the regulatory pathways, we analyzed the influence of flhDC, the master regulatory operon of the flagellum, on the yop regulon. In an flhDC mutant, the yop regulon was up-regulated. The transcription of virF and the steady-state level of the transcriptional activator VirF were enhanced. yop transcription was increased at 37 degrees C and could also be detected at a low temperature. Yop secretion was increased at 37 degrees C and occurred even at a low temperature. The Ysc secretion machinery was thus functional at room temperature in the absence of flagella, implying that in wild-type bacteria, FlhD and/or FlhC, or the product of a gene downstream of flhDC, represses the yop regulon. In agreement with this notion, increased expression of flhDC in wild-type bacteria resulted in the oversecretion of flagellins at room temperature and in decreased Yop secretion at 37 degrees C.
Figures



References
-
- Bleves, S., and G. R. Cornelis. 2000. How to survive in the host: the Yersinia lesson. Microbes Infect. 2:1451-1460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

