In vivo analysis of an essential archaeal signal recognition particle in its native host
- PMID: 12029042
- PMCID: PMC135113
- DOI: 10.1128/JB.184.12.3260-3267.2002
In vivo analysis of an essential archaeal signal recognition particle in its native host
Abstract
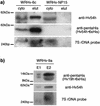
The evolutionarily conserved signal recognition particle (SRP) plays an integral role in Sec-mediated cotranslational protein translocation and membrane protein insertion, as it has been shown to target nascent secretory and membrane proteins to the bacterial and eukaryotic translocation pores. However, little is known about its function in archaea, since characterization of the SRP in this domain of life has thus far been limited to in vitro reconstitution studies of heterologously expressed archaeal SRP components identified by sequence comparisons. In the present study, the genes encoding the SRP54, SRP19, and 7S RNA homologs (hv54h, hv19h, and hv7Sh, respectively) of the genetically and biochemically tractable archaeon Haloferax volcanii were cloned, providing the tools to analyze the SRP in its native host. As part of this analysis, an hv54h knockout strain was created. In vivo characterization of this strain revealed that the archaeal SRP is required for viability, suggesting that cotranslational protein translocation is an essential process in archaea. Furthermore, a method for the purification of this SRP employing nickel chromatography was developed in H. volcanii, allowing the successful copurification of (i) Hv7Sh with a histidine-tagged Hv54h, as well as (ii) Hv54h and Hv7Sh with a histidine-tagged Hv19h. These results provide the first in vivo evidence that these components interact in archaea. Such copurification studies will provide insight into the significance of the similarities and differences of the protein-targeting systems of the three domains of life, thereby increasing knowledge about the recognition of translocated proteins in general.
Figures



Similar articles
-
The Archaeal Signal Recognition Particle: Present Understanding and Future Perspective.Curr Microbiol. 2017 Feb;74(2):284-297. doi: 10.1007/s00284-016-1167-9. Epub 2016 Nov 29. Curr Microbiol. 2017. PMID: 27900448 Review.
-
Reconstitution of the signal recognition particle of the halophilic archaeon Haloferax volcanii.Nucleic Acids Res. 2002 Oct 1;30(19):4166-75. doi: 10.1093/nar/gkf548. Nucleic Acids Res. 2002. PMID: 12364595 Free PMC article.
-
Archaea signal recognition particle shows the way.Archaea. 2010 Jun 28;2010:485051. doi: 10.1155/2010/485051. Archaea. 2010. PMID: 20672053 Free PMC article. Review.
-
Archaeal SRP RNA and SRP19 facilitate the assembly of SRP54-FtsY targeting complex.Biochem Biophys Res Commun. 2021 Aug 20;566:53-58. doi: 10.1016/j.bbrc.2021.05.087. Epub 2021 Jun 8. Biochem Biophys Res Commun. 2021. PMID: 34116357
-
Structure of the SRP19 RNA complex and implications for signal recognition particle assembly.Nature. 2002 Jun 13;417(6890):767-71. doi: 10.1038/nature00768. Epub 2002 Jun 5. Nature. 2002. PMID: 12050674
Cited by
-
Mutations in signal recognition particle SRP54 cause syndromic neutropenia with Shwachman-Diamond-like features.J Clin Invest. 2017 Nov 1;127(11):4090-4103. doi: 10.1172/JCI92876. Epub 2017 Oct 3. J Clin Invest. 2017. PMID: 28972538 Free PMC article.
-
The Archaeal Signal Recognition Particle: Present Understanding and Future Perspective.Curr Microbiol. 2017 Feb;74(2):284-297. doi: 10.1007/s00284-016-1167-9. Epub 2016 Nov 29. Curr Microbiol. 2017. PMID: 27900448 Review.
-
Functional genomic and advanced genetic studies reveal novel insights into the metabolism, regulation, and biology of Haloferax volcanii.Archaea. 2011;2011:602408. doi: 10.1155/2011/602408. Epub 2011 Nov 30. Archaea. 2011. PMID: 22190865 Free PMC article.
-
Archaeal cell surface biogenesis.FEMS Microbiol Rev. 2018 Sep 1;42(5):694-717. doi: 10.1093/femsre/fuy027. FEMS Microbiol Rev. 2018. PMID: 29912330 Free PMC article.
-
Archaeal and bacterial SecD and SecF homologs exhibit striking structural and functional conservation.J Bacteriol. 2006 Feb;188(4):1251-9. doi: 10.1128/JB.188.4.1251-1259.2006. J Bacteriol. 2006. PMID: 16452406 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

