Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible
- PMID: 12029044
- PMCID: PMC135084
- DOI: 10.1128/JB.184.12.3276-3286.2002
Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible
Abstract
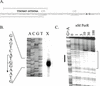
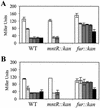
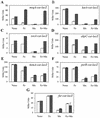
PerR is a ferric uptake repressor (Fur) homolog that functions as the central regulator of the inducible peroxide stress response in Bacillus subtilis. PerR has been previously demonstrated to regulate the mrgA, katA, ahpCF, hemAXCDBL, and zosA genes. We now demonstrate that PerR also mediates both the repression of its own gene and that of fur. Whereas PerR-mediated repression of most target genes can be elicited by either manganese or iron, repression of perR and fur is selective for manganese. Genetic studies indicate that repression of PerR regulon genes by either manganese or iron requires PerR and is generally independent of Fur. Indeed, in a fur mutant, iron-mediated repression is enhanced. Unexpectedly, repression of the fur gene by manganese appears to require both PerR and Fur, but only PerR binds to the fur regulatory region in vitro. The fur mutation appears to act indirectly by affecting cellular metal ion pools and thereby affecting PerR-mediated repression. While many components of the perR regulon are strongly induced by hydrogen peroxide, little, if any, induction of fur and perR could be demonstrated. Thus, not all components of the PerR regulon are components of the peroxide stimulon. We suggest that PerR exists in distinct metallated forms that differ in DNA target selectivity and in sensitivity to oxidation. This model is supported by the observation that the metal ion composition of the growth medium can greatly influence the transcriptional response of the various PerR regulon genes to hydrogen peroxide.
Figures



References
-
- Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. - PubMed
-
- Chen, L., and J. D. Helmann. 1995. Bacillus subtilis MrgA is a Dps(PexB) homologue: evidence for metalloregulation of an oxidative stress gene. Mol. Microbiol. 18:295-300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

