The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion
- PMID: 12032079
- PMCID: PMC126029
- DOI: 10.1093/emboj/21.11.2664
The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion
Abstract
Nuclear pore complexes (NPCs) restrict the nucleocytoplasmic flux of most macromolecules, but permit facilitated passage of nuclear transport receptors and their cargo complexes. We found that a simple hydrophobic interaction column can mimic the selectivity of NPCs surprisingly well and that nuclear transport receptors appear to be the most hydrophobic soluble proteins. This suggests that surface hydrophobicity represents a major sorting criterion of NPCs. The rate of NPC passage of cargo-receptor complexes is, however, not dominated just by properties of the receptors. We found that large cargo domains drastically hinder NPC passage and require more than one receptor molecule for rapid translocation. This argues against a rigid translocation channel and instead suggests that NPC passage involves a partitioning of the entire translocating species into a hydrophobic phase, whereby the receptor:cargo ratio determines the solubility in that permeability barrier. Finally, we show that interfering with hydrophobic interactions causes a reversible collapse of the permeability barrier of NPCs, which is consistent with the assumption that the barrier is formed by phenylalanine-rich nucleoporin repeats that attract each other through hydrophobic interactions.
Figures



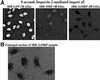
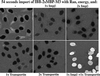


References
-
- Allen N.P., Huang,L., Burlingame,A. and Rexach,M. (2001) Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem., 276, 29268–29274. - PubMed
-
- Bayliss R., Ribbeck,K., Akin,D., Kent,H.M., Feldherr,C.M., Görlich,D. and Stewart,M. (1999) Interaction between NTF2 and xFxFG-containing nucleoporins is required to mediate nuclear import of RanGDP. J. Mol. Biol., 293, 579–593. - PubMed
-
- Bayliss R., Littlewood,T. and Stewart,M. (2000) Structural basis for the interaction between FxFG nucleoporin repeats and importin-β in nuclear trafficking. Cell, 102, 99–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

