Amyloid aggregates of the HET-s prion protein are infectious
- PMID: 12032295
- PMCID: PMC124243
- DOI: 10.1073/pnas.072199199
Amyloid aggregates of the HET-s prion protein are infectious
Abstract
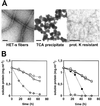
The [Het-s] infectious element of the filamentous fungus Podospora anserina is a prion. We have recently reported that recombinant HET-s protein aggregates in vitro into amyloid fibers. In vivo, the protein aggregates specifically in the [Het-s] prion strains. Here, we show that biolistic introduction of aggregated recombinant HET-s protein into fungal cells induces emergence of the [Het-s] prion with a high frequency. Thus, we demonstrate that prion infectivity can be created de novo, in vitro from recombinant protein in this system. Although the amyloid filaments formed from HET-s could transmit [Het-s] efficiently, neither the soluble form of the protein nor amorphous aggregates would do so. In addition, we have found that (i) [Het-s] infectivity correlates with the ability to convert HET-s to amyloids in vitro, (ii) [Het-s] infectivity is resistant to proteinase K digestion, and (iii) HET-s aggregates formed in vivo in [Het-s] strains have the ability to convert the recombinant protein to aggregates. Together, our data designate the HET-s amyloids as the molecular basis of [Het-s] prion propagation.
Figures


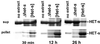
Comment in
-
Progress toward an ultimate proof of the prion hypothesis.Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9098-100. doi: 10.1073/pnas.152318899. Epub 2002 Jul 1. Proc Natl Acad Sci U S A. 2002. PMID: 12093925 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

