Inorganic polyphosphate is essential for long-term survival and virulence factors in Shigella and Salmonella spp
- PMID: 12032342
- PMCID: PMC124319
- DOI: 10.1073/pnas.112210499
Inorganic polyphosphate is essential for long-term survival and virulence factors in Shigella and Salmonella spp
Abstract
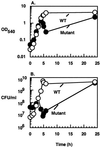
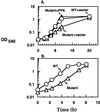
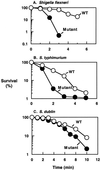
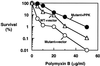
The importance of inorganic polyphosphate (poly P) and poly P kinase (PPK), the enzyme principally responsible for its synthesis, has been established previously for stationary-phase survival of Escherichia coli and virulence in Pseudomonas aeruginosa. The gene (ppk) that encodes PPK is highly conserved among many bacterial pathogens, including Shigella and Salmonella spp. In view of the phylogenetic similarity of the enteropathogens and the frequency with which virulence factors are expressed in stationary phase, the ppk gene of pathogenic Shigella flexneri, Salmonella enterica serovar Dublin, and Salmonella enterica serovar typhimurium have been cloned and deleted. In some of these mutants lacking ppk, the phenotypes included features indicative of decreased virulence such as: (i) growth defects, (ii) defective responses to stress and starvation, (iii) loss of viability, (iv) polymyxin sensitivity, (v) intolerance to acid and heat, and (vi) diminished invasiveness in epithelial cells. Thus PPK may prove, as it has with P. aeruginosa, to be an attractive target for antibiotics, with low toxicity because PPK is not found in higher eukaryotes.
Figures

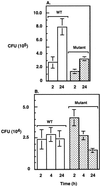
References
-
- Kulaev I S. The Biochemistry of Inorganic Polyphosphates. New York: Wiley; 1979. - PubMed
-
- Kornberg A, Rao N N, Ault-Riché D. Annu Rev Biochem. 1999;68:89–125. - PubMed
-
- Crooke E, Akiyama M, Rao N N, Kornberg A. J Biol Chem. 1994;269:6290–6295. - PubMed
-
- Tzeng C-M, Kornberg A. Mol Microbiol. 1998;29:381–382. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

