HEK293S cells have functional retinoid processing machinery
- PMID: 12034766
- PMCID: PMC2233870
- DOI: 10.1085/jgp.20018495
HEK293S cells have functional retinoid processing machinery
Abstract
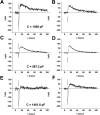
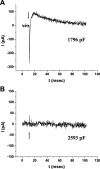
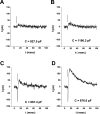
Rhodopsin activation is measured by the early receptor current (ERC), a conformation-associated charge motion, in human embryonic kidney cells (HEK293S) expressing opsins. After rhodopsin bleaching in cells loaded with 11-cis-retinal, ERC signals recover in minutes and recurrently over a period of hours by simple dark adaptation, with no added chromophore. The purpose of this study is to investigate the source of ERC signal recovery in these cells. Giant HEK293S cells expressing normal wild-type (WT)-human rod opsin (HEK293S) were regenerated by solubilized 11-cis-retinal, all-trans-retinal, or Vitamin A in darkness. ERCs were elicited by flash photolysis and measured by whole-cell recording. Visible flashes initially elicit bimodal (R(1), R(2)) ERC signals in WT-HEK293S cells loaded with 11-cis-retinal for 40 min or overnight. In contrast, cells regenerated for 40 min with all-trans-retinal or Vitamin A had negative ERCs (R(1)-like) or none at all. After these were placed in the dark overnight, ERCs with outward R(2) signals were recorded the following day. This indicates conversion of loaded Vitamin A or all-trans-retinal into cis-retinaldehyde that regenerated ground-state pigment. 4-butylaniline, an inhibitor of the mammalian retinoid cycle, reversibly suppressed recovery of the outward R(2) component from Vitamin A and 11-cis-retinal-loaded cells. These physiological findings are evidence for the presence of intrinsic retinoid processing machinery in WT-HEK293S cells similar to what occurs in the mammalian eye.
Figures




References
-
- Adler, A.J., and R.B. Edwards. 2000. Human interphotoreceptor matrix contains serum albumin and retinol-binding protein. Exp. Eye Res. 70:227–234. - PubMed
-
- Aho, A.-C., K. Donner, C. Hydén, L.O. Larsen, and T. Reuter. 1988. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature. 334:348–350. - PubMed
-
- Barry, R.J., F.J. Cañada, and R.R. Rando. 1989. Solubilization and partial purification of retinyl ester synthetase and retinoid isomerase from bovine ocular pigment epithelium. J. Biol. Chem. 264:9231–9238. - PubMed
-
- Bernstein, P.S., B.S. Fulton, and R.R. Rando. 1986. a. Mechanism of action of aromatic amines that short-circuit the visual cycle. Biochemistry. 25:3370–3377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

