The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis
- PMID: 12034770
- PMCID: PMC2173421
- DOI: 10.1083/jcb200108056
The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis
Abstract
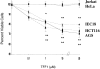
Trefoil factor (TFF)1 is synthesized and secreted by the normal stomach mucosa and by the gastrointestinal cells of injured tissues. The link between mouse TFF1 inactivation and the fully penetrant antropyloric tumor phenotype prompted the classification of TFF1 as a gastric tumor suppressor gene. Accordingly, altered expression, deletion, and/or mutations of the TFF1 gene are frequently observed in human gastric carcinomas. The present study was undertaken to address the nature of the cellular and molecular mechanisms targeted by TFF1 signalling. TFF1 effects were investigated in IEC18, HCT116, and AGS gastrointestinal cells treated with recombinant human TFF1, and in stably transfected HCT116 cells synthesizing constitutive or doxycycline-induced human TFF1. We observed that TFF1 triggers two types of cellular responses. On one hand, TFF1 lowers cell proliferation by delaying G1-S cell phase transition. This results from a TFF1-mediated increase in the levels of cyclin-dependent kinase inhibitors of both the INK4 and CIP subfamilies, leading to lower E2F transcriptional activity. On the other hand, TFF1 protects cells from chemical-, anchorage-free-, or Bad-induced apoptosis. In this process, TFF1 signalling targets the active form of caspase-9. Together, these results provide the first evidence of a dual antiproliferative and antiapoptotic role for TFF1. Similar paradoxical functions have been reported for tumor suppressor genes involved in cell differentiation, a function consistent with TFF1.
Figures

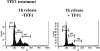
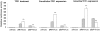


References
-
- Ashizawa, S., H. Nishizawa, M. Yamada, H. Higashi, T. Kondo, H. Ozawa, A. Kakita, and M. Hatakeyama. 2001. Collective inhibition of pRB family proteins by phosphorylation in cells with p16INK4a loss or cyclin E overexpression. J. Biol. Chem. 276:11362–11370. - PubMed
-
- Baker, S.J., S. Markowitz, E.R. Fearon, J.K. Willson, and B. Vogelstein. 1990. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 249:912–915. - PubMed
-
- Beck, S., P. Sommer, E. dos Santos Silva, N. Blin, and P. Gott. 1999. Hepatocyte nuclear factor 3 (winged helix domain) activates trefoil factor gene TFF1 through a binding motif adjacent to the TATAA box. DNA Cell Biol. 18:157–164. - PubMed
-
- Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254. - PubMed
-
- Calnan, D.P., B.R. Westley, F.E. May, D.N. Floyd, T. Marchbank, and R.J. Playford. 1999. The trefoil peptide TFF1 inhibits the growth of the human gastric adenocarcinoma cell line AGS. J. Pathol. 188:312–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

