The yeast protein Xtc1 functions as a direct transcriptional repressor
- PMID: 12034822
- PMCID: PMC117208
- DOI: 10.1093/nar/30.11.2358
The yeast protein Xtc1 functions as a direct transcriptional repressor
Abstract
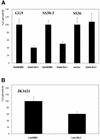
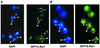
The yeast protein Xtc1 was identified as a protein that binds directly and specifically to the activation domains of acidic activators such as E2F-1, Gal4 and VP16. Additionally, it was shown to co-purify with the RNA polymerase II holoenzyme complex and it was suggested that Xtc1 functions as a regulator of transcription that modulates the response of RNA polymerase II to transcriptional activators. We have further analyzed the transcription function of Xtc1 and show that its fusion to a heterologous DNA binding domain can repress transcription of a reporter gene in vivo in an Srb10/11-dependent manner. We suggest that the presence of Xtc1 in the RNA polymerase II holoenzyme could help to recruit an Srb10-active form of the holoenzyme to target promoters. This same protein has also been implicated in mitochondrial DNA recombination, maintenance and repair. Determination of the subcellular localization using a GFP-Xtc1 fusion shows that it localizes to both the nucleus and the mitochondria in vivo, which is consistent with Xtc1 having a function in both cellular compartments.
Figures



References
-
- Lee T.I. and Young,R.A. (2000) Transcription of eukaryotic protein-coding genes. Annu. Rev. Genet., 34, 77–137. - PubMed
-
- Klein C. and Struhl,K. (1994) Increased recruitment of TATA-binding protein to the promoter by transcriptional activation domains in vivo. Science, 266, 280–282. - PubMed
-
- Ptashne M. and Gann,A. (1997) Transcriptional activation by recruitment. Nature, 386, 569–577. - PubMed
-
- Keaveney M. and Struhl,K. (1998) Activator-mediated recruitment of the RNA polymerase II machinery is the predominant mechanism for transcriptional activation in yeast. Mol. Cell, 1, 917–924. - PubMed
-
- Klages N. and Strubin,M. (1995) Stimulation of RNA polymerase II transcription initiation by recruitment of TBP in vivo. Nature, 374, 822–823. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

