Tracing the evolution of RNA structure in ribosomes
- PMID: 12034847
- PMCID: PMC117177
- DOI: 10.1093/nar/30.11.2575
Tracing the evolution of RNA structure in ribosomes
Abstract
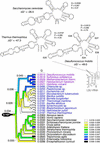
The elucidation of ribosomal structure has shown that the function of ribosomes is fundamentally confined to dynamic interactions established between the RNA components of the ribosomal ensemble. These findings now enable a detailed analysis of the evolution of ribosomal RNA (rRNA) structure. The origin and diversification of rRNA was studied here using phylogenetic tools directly at the structural level. A rooted universal tree was reconstructed from the combined secondary structures of large (LSU) and small (SSU) subunit rRNA using cladistic methods and considerations in statistical mechanics. The evolution of the complete repertoire of structural ribosomal characters was formally traced lineage-by-lineage in the tree, showing a tendency towards molecular simplification and a homogeneous reduction of ribosomal structural change with time. Character tracing revealed patterns of evolution in inter-subunit bridge contacts and tRNA-binding sites that were consistent with the proposed coupling of tRNA translocation and subunit movement. These patterns support the concerted evolution of tRNA-binding sites in the two subunits and the ancestral nature and common origin of certain structural ribosomal features, such as the peptidyl (P) site, the functional relay of the penultimate stem helix of SSU rRNA, and other structures participating in ribosomal dynamics. Overall results provide a rare insight into the evolution of ribosomal structure.
Figures





References
-
- Green R. and Noller,H.F. (1997) Ribosomes and translation. Annu. Rev. Biochem., 66, 679–716. - PubMed
-
- Cate J.H., Yusupov,M.M., Yusupova,G.Z., Earnest,T.N. and Noller,H.F. (1999) X-ray crystal structure of 70S ribosome functional complexes. Science, 285, 2095–2104. - PubMed
-
- Ban N., Nissen,P., Hansen,J., Moore,P.B. and Steitz,T.A. (2000) The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science, 289, 905–920. - PubMed
-
- Wimberly B.T., Brodersen,D.E., Clemons,W.M.,Jr, Morgan-Warren,R.J., Carter,A.P., Vonrhein,C., Hartsch,T. and Ramakrishnan,V. (2000) Structure of the 30S ribosomal subunit. Nature, 407, 306–307. - PubMed
-
- Schluenzen F., Tocilj,A., Zarivach,R., Harms,J., Gluehmann,M., Janell,D., Bashan,A., Bartels,H., Agmon,I., Franceschi,F. and Yonath,A. (2000) Structure of functionally activated small ribosomal subunit at 3.3 Å resolution. Cell, 102, 615–623. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

