Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3
- PMID: 12034882
- PMCID: PMC123085
- DOI: 10.1073/pnas.122224699
Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3
Abstract
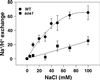
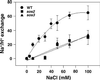
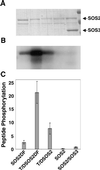
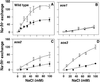
Maintaining low levels of sodium ions in the cell cytosol is critical for plant growth and development. Biochemical studies suggest that Na(+)/H(+) exchangers in the plasma membrane of plant cells contribute to cellular sodium homeostasis by transporting sodium ions out of the cell; however, these exchangers have not been identified at the molecular level. Genetic analysis has linked components of the salt overly sensitive pathway (SOS1-3) to salt tolerance in Arabidopsis thaliana. The predicted SOS1 protein sequence and comparisons of sodium ion accumulation in wild-type and sos1 plants suggest that SOS1 is involved directly in the transport of sodium ions across the plasma membrane. To demonstrate the transport capability of SOS1, we studied Na(+)/H(+)-exchange activity in wild-type and sos plants using highly purified plasma membrane vesicles. The results showed that plasma membrane Na(+)/H(+)-exchange activity was present in wild-type plants treated with 250 mM NaCl, but this transport activity was reduced by 80% in similarly treated sos1 plants. In vitro addition of activated SOS2 protein (a protein kinase) increased Na(+)/H(+)-exchange activity in salt-treated wild-type plants 2-fold relative to transport without added protein. However, the addition of activated SOS2 did not have any stimulatory effect on the exchange activity in sos1 plants. Although vesicles of sos2 and sos3 plants had reduced plasma membrane Na(+)/H(+)-exchange activity, transport activity in both increased with the addition of activated SOS2 protein. These results demonstrate that SOS1 contributes to plasma membrane Na(+)/H(+) exchange and that SOS2 and SOS3 regulate SOS1 transport activity.
Figures
References
-
- Rhoades J D, Loveday J. In: Irrigation of Agricultural Crops. Steward B A, Nielsen D R, editors. Vol. 30. Madison, WI: Am. Soc. Agronomists; 1990. pp. 1089–1142.
-
- Jacoby B. In: Handbook of Plant and Crop Stress. Pessarakli M, editor. New York: Dekker; 1999. pp. 97–123.
-
- Hasegawa P M, Bressan R A, Zhu J-K, Bohnert H J. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:463–499. - PubMed
-
- Blumwald E. Curr Opin Cell Biol. 2000;12:431–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

