All-trans-retinal shuts down rod cyclic nucleotide-gated ion channels: a novel role for photoreceptor retinoids in the response to bright light?
- PMID: 12034887
- PMCID: PMC123074
- DOI: 10.1073/pnas.122681899
All-trans-retinal shuts down rod cyclic nucleotide-gated ion channels: a novel role for photoreceptor retinoids in the response to bright light?
Abstract
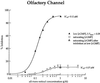
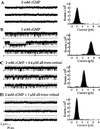
In retinal rods, light-induced isomerization of 11-cis-retinal to all-trans-retinal within rhodopsin triggers an enzyme cascade that lowers the concentration of cGMP. Consequently, cyclic nucleotide-gated (CNG) ion channels close, generating the first electrical response to light. After isomerization, all-trans-retinal dissociates from rhodopsin. We now show that all-trans-retinal directly and markedly inhibits cloned rod CNG channels in excised patches. 11-cis-retinal and all-trans-retinol also inhibited the channels, but at somewhat higher concentrations. Single-channel analysis suggests that all-trans-retinal reduces average open probability of rod CNG channels by inactivating channels for seconds at a time. At physiological cGMP levels, all-trans-retinal inhibited in the nanomolar range. Our results suggest that all-trans-retinal may be a potent regulator of the channel in rods during the response to bright light, when there is a large surge in the concentration of all-trans-retinal.
Figures




Similar articles
-
Defining the retinoid binding site in the rod cyclic nucleotide-gated channel.J Gen Physiol. 2005 Nov;126(5):453-60. doi: 10.1085/jgp.200509387. Epub 2005 Oct 17. J Gen Physiol. 2005. PMID: 16230468 Free PMC article.
-
All-trans-retinal is a closed-state inhibitor of rod cyclic nucleotide-gated ion channels.J Gen Physiol. 2004 May;123(5):521-31. doi: 10.1085/jgp.200409011. Epub 2004 Apr 12. J Gen Physiol. 2004. PMID: 15078915 Free PMC article.
-
Cyclic nucleotide-gated ion channels in rod photoreceptors are protected from retinoid inhibition.J Gen Physiol. 2006 Oct;128(4):473-85. doi: 10.1085/jgp.200609619. J Gen Physiol. 2006. PMID: 17001087 Free PMC article.
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
Cyclic nucleotide-gated channels in visual and olfactory transduction.Biophys Chem. 1995 Aug;55(3):185-96. doi: 10.1016/0301-4622(94)00153-b. Biophys Chem. 1995. PMID: 7542935 Review.
Cited by
-
The pharmacology of cyclic nucleotide-gated channels: emerging from the darkness.Curr Pharm Des. 2006;12(28):3597-613. doi: 10.2174/138161206778522100. Curr Pharm Des. 2006. PMID: 17073662 Free PMC article. Review.
-
Signalling beyond photon absorption: extracellular retinoids and growth factors modulate rod photoreceptor sensitivity.J Physiol. 2016 Apr 1;594(7):1841-54. doi: 10.1113/JP271650. Epub 2016 Jan 23. J Physiol. 2016. PMID: 26691896 Free PMC article.
-
Defining the retinoid binding site in the rod cyclic nucleotide-gated channel.J Gen Physiol. 2005 Nov;126(5):453-60. doi: 10.1085/jgp.200509387. Epub 2005 Oct 17. J Gen Physiol. 2005. PMID: 16230468 Free PMC article.
-
All-trans-retinal is a closed-state inhibitor of rod cyclic nucleotide-gated ion channels.J Gen Physiol. 2004 May;123(5):521-31. doi: 10.1085/jgp.200409011. Epub 2004 Apr 12. J Gen Physiol. 2004. PMID: 15078915 Free PMC article.
-
Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition.Am J Physiol Lung Cell Mol Physiol. 2018 Jan 1;314(1):L93-L106. doi: 10.1152/ajplung.00091.2017. Epub 2017 Sep 7. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 28882814 Free PMC article.
References
-
- Broillet M C, Firestein S. Ann NY Acad Sci. 1999;868:730–740. - PubMed
-
- Finn J T, Grunwald M E, Yau K-W. Annu Rev Physiol. 1996;58:395–426. - PubMed
-
- Kaupp U B. Curr Opin Neurobiol. 1995;5:434–442. - PubMed
-
- Molday R S, Hsu Y-T. Behav Brain Sci. 1995;18:441–451.
-
- Richards M J, Gordon S E. Biochemistry. 2000;39:14003–14011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

