Extensive interallelic polymorphisms drive meiotic recombination into a crossover pathway
- PMID: 12034905
- PMCID: PMC150615
- DOI: 10.1105/tpc.001271
Extensive interallelic polymorphisms drive meiotic recombination into a crossover pathway
Abstract
Recombinants isolated from most meiotic intragenic recombination experiments in maize, but not in yeast, are borne principally on crossover chromosomes. This excess of crossovers is not explained readily by the canonical double-strand break repair model of recombination, proposed to account for a large body of yeast data, which predicts that crossovers (COs) and noncrossovers (NCOs) should be recovered equally. An attempt has been made here to identify general rules governing the recovery of the CO and NCO classes of intragenic recombinants in maize. Recombination was analyzed in bz heterozygotes between a variety of mutations derived from the same or different progenitor alleles. The mutations include point mutations, transposon insertions, and transposon excision footprints. Consequently, the differences between the bz heteroalleles ranged from just two nucleotides to many nucleotides, indels, and insertions. In this article, allelic pairs differing at only two positions are referred to as dimorphic to distinguish them from polymorphic pairs, which differ at multiple positions. The present study has revealed the following effects at these bz heteroalleles: (1) recombination between polymorphic heteroalleles produces mostly CO chromosomes; (2) recombination between dimorphic heteroalleles produces both CO and NCO chromosomes, in ratios apparently dependent on the nature of the heteroalleles; and (3) in dimorphic heterozygotes, the two NCO classes are recovered in approximately equal numbers when the two mutations are point mutations but not when one or both mutations are insertions. These observations are discussed in light of a recent version of the double-strand break repair model of recombination that postulates separate pathways for the formation of CO and NCO products.
Figures
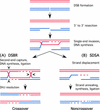
References
-
- Allers, T., and Lichten, M. (2001). Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106, 47–57. - PubMed
-
- Baker, S.M., Plug, A.W., Prolla, T.A., Bronner, C.E., Harris, A.C., Yao, X., Christie, D.M., Monell, C., Arnheim, N., Bradley, A., Ashley, T., and Liskay, R.M. (1996). Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat. Genet. 13, 336–342. - PubMed
-
- Dooner, H.K. (1998). On the possible occurrence of conversion polarity at the bronze locus. Plant Cell 10, 646–648.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

