Crescent bodies of Parachlamydia acanthamoeba and its life cycle within Acanthamoeba polyphaga: an electron micrograph study
- PMID: 12039769
- PMCID: PMC123927
- DOI: 10.1128/AEM.68.6.3076-3084.2002
Crescent bodies of Parachlamydia acanthamoeba and its life cycle within Acanthamoeba polyphaga: an electron micrograph study
Abstract
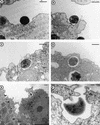
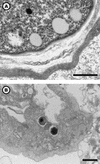
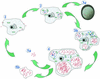
Parachlamydiaceae are endosymbionts of free-living amoeba first identified in 1997. Two developmental stages, elementary and reticulate bodies, were observed; however, their localization and proportions according to culture condition and duration remain unknown. The life cycle of Parachlamydia acanthamoeba within Acanthamoeba polyphaga was studied by transmission electron microscopy of 8-, 36-, and 144-h coculture. Morphometry and quantification were performed using SAMBA software. The elementary body, the predominant stage within the amoebae, was located mainly within their vacuoles. The multiplication of Parachlamydia bacteria by binary fission of reticulate bodies was independently associated with culture in PYG broth (odds ratio [OR] = 4.4; 95% confidence interval [CI], 1.55 to 12.46) and with the presence of reticulate bodies within the amoebae (OR = 2.10; 95% CI, 1.53 to 2.89). A third developmental stage was observed, the crescent body. Its presence outside and inside the amoebae was associated mainly with prolonged incubation time (OR = 3.98; 95% CI, 1.49 to 10.68, and OR = 5.98; 95% CI, 1.75 to 20.4, respectively). Elementary and crescent bodies were released into the extracellular medium within vesicles or after amoebal lysis. For both, phagocytosis was their mode of entry. This electron micrograph study revealed another infective developmental stage, the crescent body, and provided quantitative analysis of the life cycle of P. acanthamoeba within A. polyphaga.
Figures




References
-
- Birtles, R. J., T. J. Rowbotham, D. Raoult, and T. G. Harrison. 1996. Phylogenetic diversity of intra-amoebal legionellae as revealed by 16S rRNA gene sequence comparison. Microbiology 142:3525-3530. - PubMed
-
- Birtles, R. J., T. J. Rowbotham, C. Storey, T. J. Marrie, and D. Raoult. 1997. Chlamydia-like obligate parasite of free-living amoebae. Lancet 349:925-926. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

