Rapid detection and enumeration of Naegleria fowleri in surface waters by solid-phase cytometry
- PMID: 12039772
- PMCID: PMC123984
- DOI: 10.1128/AEM.68.6.3102-3107.2002
Rapid detection and enumeration of Naegleria fowleri in surface waters by solid-phase cytometry
Abstract
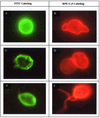
A new method for the rapid and accurate detection of pathogenic Naegleria fowleri amoebae in surface environmental water was developed. The method is based on an immunofluorescent assay combined with detection by solid-phase cytometry. In this study we developed and compared two protocols using different reporter systems conjugated to antibodies. The monoclonal antibody Ac5D12 was conjugated with biotin and horseradish peroxidase, and the presence of cells was revealed with streptavidin conjugated to both R-phycoerythrin and cyanine Cy5 (RPE-Cy5) and tyramide-fluorescein isothiocyanate, respectively. The RPE-Cy5 protocol was the most efficient protocol and allowed the detection of both trophozoite and cyst forms in water. The direct counts obtained by this new method were not significantly different from those obtained by the traditional culture approach, and results were provided within 3 h. The sensitivity of the quantitative method is 200 cells per liter. The limit is due only to the filtration capacity of the membrane used.
Figures

References
-
- Cable, B. L., and D. T. John. 1986. Conditions for maximum enflagellation in Naegleria fowleri. J. Protozool. 33:467-472. - PubMed
-
- Carter, R. F. 1970. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis and of the experimental pathological changes induced by it. J. Pathol. 100:217-244. - PubMed
-
- Champsaur, H. 1996. Méthodes générales d'examen bactériologique des eaux, p. 755-756. In J. Rodier (ed.), L'analyse de l'eau. Dunod, Paris, France.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

