An Otx-related homeodomain protein binds an LHbeta promoter element important for activation during gonadotrope maturation
- PMID: 12040015
- PMCID: PMC2932471
- DOI: 10.1210/mend.16.6.0841
An Otx-related homeodomain protein binds an LHbeta promoter element important for activation during gonadotrope maturation
Abstract
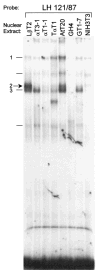
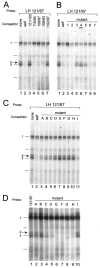
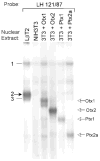
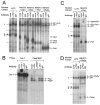
The hormone-secreting cell types of the anterior pituitary differentiate in a specific spatial and temporal manner. The alpha-subunit of the glycoprotein hormones appears at embryonic d 11.5 in the mouse, followed by steroidogenic factor-1, which distinguishes the gonadotrope progenitor cells, around embryonic d 14. Gonadotrope maturation is marked by the onset of LHbeta-gene expression 2 d later. The alphaT3-1 and LbetaT2 immortalized mouse pituitary cell lines correspond to these later sequential stages of gonadotrope differentiation. In addition to the early markers of the gonadotrope lineage present in alphaT3-1 cells, LbetaT2 cells also express markers of a mature gonadotrope, including LHbeta and FSHbeta. Using transient transfections to compare expression among gonadotrope and nongonadotrope-derived cell types, we show that the rat 1.8-kb LHbeta promoter directs reporter gene expression specifically to the mature gonadotrope LbetaT2 cell line. Promoter truncation and mutagenesis analyses indicate that the homeodomain (HD) element located at approximately -100 bp relative to the transcriptional start site is essential for this selectivity to LbetaT2 cells when compared with alphaT3-1 cells. In EMSAs, this HD site binds a protein present in LbetaT2 but not other gonadotrope-derived cells. Antibody supershift and competition experiments indicate that this LbetaT2 nuclear protein is a K50 HD protein related to the Otx family, though it is not a known pituitary homeobox transcription factor protein. These studies indicate a role for a novel Otx-related HD protein in gonadotrope maturation during development.
Figures




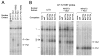
Similar articles
-
Activin regulates luteinizing hormone beta-subunit gene expression through Smad-binding and homeobox elements.Mol Endocrinol. 2005 Oct;19(10):2610-23. doi: 10.1210/me.2005-0047. Epub 2005 Jun 16. Mol Endocrinol. 2005. PMID: 15961509 Free PMC article.
-
Cell-specific transcriptional regulation of follicle-stimulating hormone-beta by activin and gonadotropin-releasing hormone in the LbetaT2 pituitary gonadotrope cell model.Endocrinology. 2001 Jun;142(6):2284-95. doi: 10.1210/endo.142.6.8185. Endocrinology. 2001. PMID: 11356674
-
Msx1 homeodomain protein represses the αGSU and GnRH receptor genes during gonadotrope development.Mol Endocrinol. 2013 Mar;27(3):422-36. doi: 10.1210/me.2012-1289. Epub 2013 Jan 31. Mol Endocrinol. 2013. PMID: 23371388 Free PMC article.
-
The promoter of the rat gonadotropin-releasing hormone receptor gene directs the expression of the human placental alkaline phosphatase reporter gene in gonadotrope cells in the anterior pituitary gland as well as in multiple extrapituitary tissues.Endocrinology. 2004 Feb;145(2):983-93. doi: 10.1210/en.2003-0881. Epub 2003 Oct 30. Endocrinology. 2004. PMID: 14592958
-
[An ambiguous role of steroidogenic factor 1 in the rat GnRH receptor gene expression. Lessons from transgenic mice].J Soc Biol. 2004;198(1):73-9. J Soc Biol. 2004. PMID: 15146959 Review. French.
Cited by
-
Hormones in synergy: regulation of the pituitary gonadotropin genes.Mol Cell Endocrinol. 2010 Jan 27;314(2):192-203. doi: 10.1016/j.mce.2009.09.003. Epub 2009 Sep 10. Mol Cell Endocrinol. 2010. PMID: 19747958 Free PMC article. Review.
-
Novel forms of Paired-like homeodomain transcription factor 2 (PITX2): generation by alternative translation initiation and mRNA splicing.BMC Mol Biol. 2008 Mar 28;9:31. doi: 10.1186/1471-2199-9-31. BMC Mol Biol. 2008. PMID: 18373856 Free PMC article.
-
NeuroD1 and Mash1 temporally regulate GnRH receptor gene expression in immortalized mouse gonadotrope cells.Mol Cell Endocrinol. 2008 Nov 25;295(1-2):106-14. doi: 10.1016/j.mce.2008.07.017. Epub 2008 Aug 6. Mol Cell Endocrinol. 2008. PMID: 18760324 Free PMC article.
-
Androgens, progestins, and glucocorticoids induce follicle-stimulating hormone beta-subunit gene expression at the level of the gonadotrope.Mol Endocrinol. 2006 Sep;20(9):2062-79. doi: 10.1210/me.2005-0316. Epub 2006 May 4. Mol Endocrinol. 2006. PMID: 16675544 Free PMC article.
-
Activin and glucocorticoids synergistically activate follicle-stimulating hormone beta-subunit gene expression in the immortalized LbetaT2 gonadotrope cell line.Endocrinology. 2007 Feb;148(2):762-73. doi: 10.1210/en.2006-0952. Epub 2006 Nov 2. Endocrinology. 2007. PMID: 17082263 Free PMC article.
References
-
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. - PubMed
-
- Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329. - PubMed
-
- Graham KE, Nusser KD, Low MJ. LβT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to activin A. J Endocrinol. 1999;162:R1–R5. - PubMed
-
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang H-J, Miller WL, Mellon PL. Cell-specific transcriptional regulation of FSHβ by activin and GnRH in the LβT2 pituitary gonadotrope cell model. Endocrinology. 2001;142:2284–2295. - PubMed
-
- Halvorson LM, Ito M, Jameson JL, Chin WW. Steroidogenic factor-1 and early growth response protein 1 act through two composite DNA binding sites to regulate luteinizing hormone β-subunit gene expression. J Biol Chem. 1998;273:14712–14720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

