Transport of Neuronal BC1 RNA in Mauthner Axons
- PMID: 12040034
- PMCID: PMC1828540
- DOI: 10.1523/JNEUROSCI.22-11-04293.2002
Transport of Neuronal BC1 RNA in Mauthner Axons
Abstract
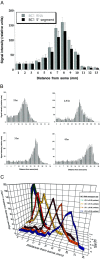
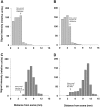
In neurons, localized RNAs have been identified in dendrites and axons; however, RNA transport in axons remains poorly understood. Here we analyzed axonal RNA transport in goldfish Mauthner neurons in vivo. BC1 RNA, a noncoding RNA polymerase III transcript that is targeted to dendrites in neurons of the rodent nervous system, was used as a probe for axonal RNA transport. Somata of Mauthner neurons were microinjected with various RNAs. Full-length BC1 RNA, but not control RNAs of similar length, was targeted to both axons and dendrites of Mauthner neurons. BC1 RNA was transported in the form of a rapidly advancing wave front that progressed along axons, in a microtubule-dependent manner, at a rate of 2 micrometer/sec. Whereas a BC1 5' segment of 65 nucleotides was transported to axons and dendrites in a way indistinguishable from full-length BC1 RNA, a BC1 3' segment of 60 nucleotides did not enter Mauthner cell processes to any significant extent. In the wake of the wave advancing through the axon, BC1 RNA was found localized to discrete, spatially delimited domains at the axonal surface. Such demarcated cortical concentrations of BC1 RNA could not be observed after disruption of F-actin organization in the axon. It is concluded that the specific delivery of BC1 RNA to spatially defined axonal target sites is a two-step process that requires the sequential participation of microtubules for long-range axial transport and of actin filaments for local radial transfer and focal accumulation in cortical domains.
Figures



Similar articles
-
Neuronal BC1 RNA: microtubule-dependent dendritic delivery.J Mol Biol. 2006 Mar 10;356(5):1118-23. doi: 10.1016/j.jmb.2005.11.090. Epub 2005 Dec 19. J Mol Biol. 2006. PMID: 16412460 Free PMC article.
-
RNA transport in dendrites: a cis-acting targeting element is contained within neuronal BC1 RNA.J Neurosci. 1997 Jun 15;17(12):4722-33. doi: 10.1523/JNEUROSCI.17-12-04722.1997. J Neurosci. 1997. PMID: 9169532 Free PMC article.
-
Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons.J Comp Neurol. 2000 Nov 20;427(3):351-61. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. J Comp Neurol. 2000. PMID: 11054698
-
Neuronal BC1 RNA: intracellular transport and activity-dependent modulation.Results Probl Cell Differ. 2001;34:129-38. doi: 10.1007/978-3-540-40025-7_8. Results Probl Cell Differ. 2001. PMID: 11288672 Review. No abstract available.
-
Axonal mRNAs: functional significance in vertebrates and invertebrates.J Neurocytol. 2000 Nov-Dec;29(11-12):783-91. doi: 10.1023/a:1010987206526. J Neurocytol. 2000. PMID: 11466470 Review.
Cited by
-
Neuronal sub-compartmentalization: a strategy to optimize neuronal function.Biol Rev Camb Philos Soc. 2019 Jun;94(3):1023-1037. doi: 10.1111/brv.12487. Epub 2019 Jan 4. Biol Rev Camb Philos Soc. 2019. PMID: 30609235 Free PMC article. Review.
-
Spatial code recognition in neuronal RNA targeting: role of RNA-hnRNP A2 interactions.J Cell Biol. 2011 Aug 8;194(3):441-57. doi: 10.1083/jcb.201010027. Epub 2011 Aug 1. J Cell Biol. 2011. PMID: 21807882 Free PMC article.
-
Redistribution of syntaxin mRNA in neuronal cell bodies regulates protein expression and transport during synapse formation and long-term synaptic plasticity.J Neurosci. 2003 Mar 1;23(5):1804-15. doi: 10.1523/JNEUROSCI.23-05-01804.2003. J Neurosci. 2003. PMID: 12629184 Free PMC article.
-
Molecular mechanisms behind mRNA localization in axons.Open Biol. 2020 Sep;10(9):200177. doi: 10.1098/rsob.200177. Epub 2020 Sep 23. Open Biol. 2020. PMID: 32961072 Free PMC article. Review.
-
Of social molecules: The interactive assembly of ASH1 mRNA-transport complexes in yeast.RNA Biol. 2014;11(8):998-1009. doi: 10.4161/rna.29946. Epub 2014 Oct 31. RNA Biol. 2014. PMID: 25482892 Free PMC article. Review.
References
-
- Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog Neurobiol. 2000;62:1–62. - PubMed
-
- Banker K, Goslin K. Culturing nerve Cells. MIT; Cambridge, MA: 1998.
-
- Barker LF. The nervous system and its constituent neurons. D. Appleton and Company; New York: 1899.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
