Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells
- PMID: 12040035
- PMCID: PMC6758793
- DOI: 10.1523/JNEUROSCI.22-11-04302.2002
Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells
Abstract
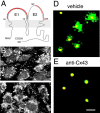
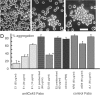
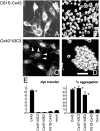
A hallmark of astrocytic tumors is their infiltrative nature. Although their aggressive and typically widespread dispersal in the adult brain differs fundamentally from that of other brain tumors, little is known about their cellular basis. Astrocytic tumors express the gap junction protein connexin 43 (Cx43), and we show here that Cx43 expression induced the morphological transformation of glioma cells into an epithelial phenotype. In a short-term aggregation assay, Cx43 expression was associated with a several-fold increase in the competence of glioma cells to aggregate. Antibodies directed against the extracellular domain of Cx43 restored the connexin-deficient phenotype, as manifested by a dose-dependent reduction in aggregation. Apart from their role in gap junction formation, connexins may therefore be considered a distinct class of membrane proteins with adhesive properties. Moreover, implanted Cx43-expressing glioma cells established functional gap junction channels with host astrocytes and dispersed through a substantially greater volume of brain parenchyma than mock- and mutant Cx43-transfected sister cells. Cx43 expression therefore may modulate not only the adhesion of astrocytes to one another, but the spread of glial tumor cells throughout astrocytic syncytia. These observations widen our concept of the potential interactions between tumor cells and their surroundings and suggest that both connexin proteins and their derived gap junctions are critical determinants of the invasiveness of central gliomas.
Figures



Similar articles
-
Increased invasive capacity of connexin43-overexpressing malignant glioma cells.J Neurosurg. 2003 Dec;99(6):1039-46. doi: 10.3171/jns.2003.99.6.1039. J Neurosurg. 2003. PMID: 14705732
-
Communication between malignant glioma cells and vascular endothelial cells through gap junctions.J Neurosurg. 2003 Apr;98(4):846-53. doi: 10.3171/jns.2003.98.4.0846. J Neurosurg. 2003. PMID: 12691411
-
Dose-dependent differential upregulation of CCN1/Cyr61 and CCN3/NOV by the gap junction protein Connexin43 in glioma cells.J Cell Biochem. 2008 Apr 15;103(6):1772-82. doi: 10.1002/jcb.21571. J Cell Biochem. 2008. PMID: 18004727
-
Connexin 43 (Cx43) in cancer: Implications for therapeutic approaches via gap junctions.Cancer Lett. 2019 Feb 1;442:439-444. doi: 10.1016/j.canlet.2018.10.043. Epub 2018 Nov 22. Cancer Lett. 2019. PMID: 30472182 Review.
-
Common mechanisms linking connexin43 to neural progenitor cell migration and glioma invasion.Semin Cell Dev Biol. 2016 Feb;50:59-66. doi: 10.1016/j.semcdb.2015.12.008. Epub 2015 Dec 17. Semin Cell Dev Biol. 2016. PMID: 26706148 Review.
Cited by
-
Cell coupling mediated by connexin 26 selectively contributes to reduced adhesivity and increased migration.J Cell Sci. 2016 Dec 1;129(23):4399-4410. doi: 10.1242/jcs.185017. Epub 2016 Oct 24. J Cell Sci. 2016. PMID: 27777264 Free PMC article.
-
The role of gap junction channels during physiologic and pathologic conditions of the human central nervous system.J Neuroimmune Pharmacol. 2012 Sep;7(3):499-518. doi: 10.1007/s11481-012-9352-5. Epub 2012 Mar 23. J Neuroimmune Pharmacol. 2012. PMID: 22438035 Free PMC article. Review.
-
Connexin 30 Deficiency Ameliorates Disease Progression at the Early Phase in a Mouse Model of Amyotrophic Lateral Sclerosis by Suppressing Glial Inflammation.Int J Mol Sci. 2022 Dec 16;23(24):16046. doi: 10.3390/ijms232416046. Int J Mol Sci. 2022. PMID: 36555685 Free PMC article.
-
Assembly of connexin43 into gap junctions is regulated differentially by E-cadherin and N-cadherin in rat liver epithelial cells.Mol Biol Cell. 2010 Dec;21(23):4089-107. doi: 10.1091/mbc.E10-05-0403. Epub 2010 Sep 29. Mol Biol Cell. 2010. PMID: 20881055 Free PMC article.
-
What is hidden in the pannexin treasure trove: the sneak peek and the guesswork.J Cell Mol Med. 2006 Jul-Sep;10(3):613-34. doi: 10.1111/j.1582-4934.2006.tb00424.x. J Cell Mol Med. 2006. PMID: 16989724 Free PMC article. Review.
References
-
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. - PubMed
-
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long range glial signaling. Science. 1990;247:470–474. - PubMed
-
- Dezawa M, Nagano T. Immunohistochemical localization of cell adhesion molecules and cell–cell contact proteins during regeneration of the rat optic nerve induced by sciatic nerve autotransplantation. Anat Rec. 1996;246:114–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
