Domain 2 of Drosophila para voltage-gated sodium channel confers insect properties to a rat brain channel
- PMID: 12040042
- PMCID: PMC6758777
- DOI: 10.1523/JNEUROSCI.22-11-04364.2002
Domain 2 of Drosophila para voltage-gated sodium channel confers insect properties to a rat brain channel
Abstract
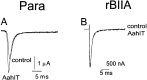
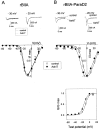
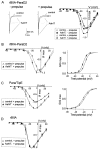
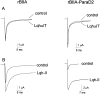
The ability of the excitatory anti-insect-selective scorpion toxin AahIT (Androctonus australis hector) to exclusively bind to and modify the insect voltage-gated sodium channel (NaCh) makes it a unique tool to unravel the structural differences between mammalian and insect channels, a prerequisite in the design of selective pesticides. To localize the insect NaCh domain that binds AahIT, we constructed a chimeric channel composed of rat brain NaCh alpha-subunit (rBIIA) in which domain-2 (D2) was replaced by that of Drosophila Para (paralytic temperature-sensitive). The choice of D2 was dictated by the similarity between AahIT and scorpion beta-toxins pertaining to both their binding and action and the essential role of D2 in the beta-toxins binding site on mammalian channels. Expression of the chimera rBIIA-ParaD2 in Xenopus oocytes gave rise to voltage-gated and TTX-sensitive NaChs that, like rBIIA, were sensitive to scorpion alpha-toxins and regulated by the auxiliary subunit beta(1) but not by the insect TipE. Notably, like Drosophila Para/TipE, but unlike rBIIA/beta(1), the chimera gained sensitivity to AahIT, indicating that the phyletic selectivity of AahIT is conferred by the insect NaCh D2. Furthermore, the chimera acquired additional insect channel properties; its activation was shifted to more positive potentials, and the effect of alpha-toxins was potentiated. Our results highlight the key role of D2 in the selective recognition of anti-insect excitatory toxins and in the modulation of NaCh gating. We also provide a methodological approach to the study of ion channels that are difficult to express in model expression systems.
Figures

Similar articles
-
beta-Scorpion toxin modifies gating transitions in all four voltage sensors of the sodium channel.J Gen Physiol. 2007 Sep;130(3):257-68. doi: 10.1085/jgp.200609719. Epub 2007 Aug 13. J Gen Physiol. 2007. PMID: 17698594 Free PMC article.
-
An 'Old World' scorpion beta-toxin that recognizes both insect and mammalian sodium channels.Eur J Biochem. 2003 Jun;270(12):2663-70. doi: 10.1046/j.1432-1033.2003.03643.x. Eur J Biochem. 2003. PMID: 12787033
-
Differential effects of five 'classical' scorpion beta-toxins on rNav1.2a and DmNav1 provide clues on species-selectivity.Toxicol Appl Pharmacol. 2007 Jan 1;218(1):45-51. doi: 10.1016/j.taap.2006.10.009. Epub 2006 Oct 14. Toxicol Appl Pharmacol. 2007. PMID: 17118417 Free PMC article.
-
Insect-selective spider toxins targeting voltage-gated sodium channels.Toxicon. 2007 Mar 15;49(4):490-512. doi: 10.1016/j.toxicon.2006.11.027. Epub 2006 Dec 5. Toxicon. 2007. PMID: 17223149 Review.
-
The differential preference of scorpion alpha-toxins for insect or mammalian sodium channels: implications for improved insect control.Toxicon. 2007 Mar 15;49(4):452-72. doi: 10.1016/j.toxicon.2006.11.016. Epub 2006 Nov 28. Toxicon. 2007. PMID: 17215013 Review.
Cited by
-
Miniaturization of scorpion beta-toxins uncovers a putative ancestral surface of interaction with voltage-gated sodium channels.J Biol Chem. 2008 May 30;283(22):15169-76. doi: 10.1074/jbc.M801229200. Epub 2008 Mar 13. J Biol Chem. 2008. PMID: 18339620 Free PMC article.
-
Molecular requirements for recognition of brain voltage-gated sodium channels by scorpion alpha-toxins.J Biol Chem. 2009 Jul 31;284(31):20684-91. doi: 10.1074/jbc.M109.021303. Epub 2009 Jun 9. J Biol Chem. 2009. PMID: 19509294 Free PMC article.
-
Elucidation of the molecular basis of selective recognition uncovers the interaction site for the core domain of scorpion alpha-toxins on sodium channels.J Biol Chem. 2011 Oct 7;286(40):35209-17. doi: 10.1074/jbc.M111.259507. Epub 2011 Aug 8. J Biol Chem. 2011. PMID: 21832067 Free PMC article.
-
Convergent evolution of sodium ion selectivity in metazoan neuronal signaling.Cell Rep. 2012 Aug 30;2(2):242-8. doi: 10.1016/j.celrep.2012.06.016. Epub 2012 Jul 26. Cell Rep. 2012. PMID: 22854023 Free PMC article.
-
The insecticidal potential of venom peptides.Cell Mol Life Sci. 2013 Oct;70(19):3665-93. doi: 10.1007/s00018-013-1315-3. Epub 2013 Mar 23. Cell Mol Life Sci. 2013. PMID: 23525661 Free PMC article. Review.
References
-
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn J, Catterall WA, Lester HA, Davidson N, Dunn RJ. A rat brain Na+ channel α subunit with novel gating properties. Neuron. 1988;1:449–461. - PubMed
-
- Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992;72:S15–S48. - PubMed
-
- Catterall WA. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
