Regulation of neurotransmitter release by synapsin III
- PMID: 12040043
- PMCID: PMC6758821
- DOI: 10.1523/JNEUROSCI.22-11-04372.2002
Regulation of neurotransmitter release by synapsin III
Abstract
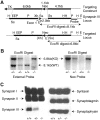
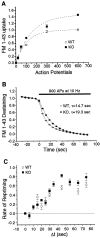
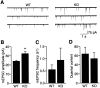
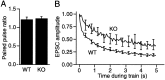
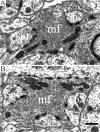
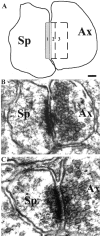
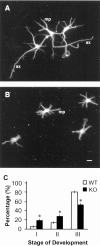
Synapsin III is the most recently identified member of the synapsin family, a group of synaptic vesicle proteins that play essential roles in neurotransmitter release and neurite outgrowth. Here, through the generation and analysis of synapsin III knock-out mice, we demonstrate that synapsin III regulates neurotransmitter release in a manner that is distinct from that of synapsin I or synapsin II. In mice lacking synapsin III, the size of the recycling pool of synaptic vesicles was increased, and synaptic depression was reduced. The number of vesicles that fuse per action potential was similar between synapsin III knock-out and wild-type mice, and there was no change in the quantal content of EPSCs; however, IPSCs were greatly reduced in synapsin III-deficient neurons. The density and distribution of synaptic vesicles in presynaptic terminals did not appear to be different in synapsin III knock-out mice in comparison to wild-type littermates. In addition to the changes in neurotransmitter release, we observed a specific delay in axon outgrowth in cultured hippocampal neurons from synapsin III knock-out mice. Our data indicate that synapsin III plays unique roles both in early axon outgrowth and in the regulation of synaptic vesicle trafficking.
Figures
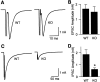
Similar articles
-
Different presynaptic roles of synapsins at excitatory and inhibitory synapses.J Neurosci. 2004 Dec 15;24(50):11368-80. doi: 10.1523/JNEUROSCI.3795-04.2004. J Neurosci. 2004. PMID: 15601943 Free PMC article.
-
Synapsin Isoforms Regulating GABA Release from Hippocampal Interneurons.J Neurosci. 2016 Jun 22;36(25):6742-57. doi: 10.1523/JNEUROSCI.0011-16.2016. J Neurosci. 2016. PMID: 27335405 Free PMC article.
-
Synapsin selectively controls the mobility of resting pool vesicles at hippocampal terminals.J Neurosci. 2012 Mar 21;32(12):3969-80. doi: 10.1523/JNEUROSCI.5058-11.2012. J Neurosci. 2012. PMID: 22442064 Free PMC article.
-
The synaptic vesicle cycle revisited.Neuron. 2000 Nov;28(2):317-20. doi: 10.1016/s0896-6273(00)00109-4. Neuron. 2000. PMID: 11144340 Review. No abstract available.
-
The synapsin cycle: a view from the synaptic endocytic zone.J Neurosci Res. 2007 Sep;85(12):2648-56. doi: 10.1002/jnr.21176. J Neurosci Res. 2007. PMID: 17455288 Review.
Cited by
-
The different clinical facets of SYN1-related neurodevelopmental disorders.Front Cell Dev Biol. 2022 Dec 8;10:1019715. doi: 10.3389/fcell.2022.1019715. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36568968 Free PMC article.
-
O-GlcNAc transferase OGT-1 and the ubiquitin ligase EEL-1 modulate seizure susceptibility in C. elegans.PLoS One. 2021 Nov 19;16(11):e0260072. doi: 10.1371/journal.pone.0260072. eCollection 2021. PLoS One. 2021. PMID: 34797853 Free PMC article.
-
Synapsin II and calcium regulate vesicle docking and the cross-talk between vesicle pools at the mouse motor terminals.J Physiol. 2008 Oct 1;586(19):4649-73. doi: 10.1113/jphysiol.2008.154666. Epub 2008 Jul 31. J Physiol. 2008. PMID: 18669537 Free PMC article.
-
H3K4 tri-methylation in synapsin genes leads to different expression patterns in bipolar disorder and major depression.Int J Neuropsychopharmacol. 2013 Mar;16(2):289-99. doi: 10.1017/S1461145712000363. Epub 2012 May 9. Int J Neuropsychopharmacol. 2013. PMID: 22571925 Free PMC article.
-
Lighting a path: genetic studies pinpoint neurodevelopmental mechanisms in autism and related disorders.Dialogues Clin Neurosci. 2012 Sep;14(3):239-52. Dialogues Clin Neurosci. 2012. PMID: 23226950 Free PMC article. Review.
References
-
- Bahler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–707. - PubMed
-
- Bahler M, Benfenati F, Valtorta F, Greengard P. The synapsins and the regulation of synaptic function. BioEssays. 1990;12:259–263. - PubMed
-
- Benfenati F, Valtorta F, Bahler M, Greengard P. Synapsin I, a neuron-specific phosphoprotein interacting with small synaptic vesicles and F-actin. Cell Biol Int Rep. 1989;13:1007–1021. - PubMed
-
- Benfenati F, Valtorta F, Rubenstein JL, Gorelick FS, Greengard P, Czernik AJ. Synaptic vesicle-associated Ca2+/calmodulin-dependent protein kinase II is a binding protein for synapsin I. Nature. 1992;359:417–420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
