Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse
- PMID: 12040050
- PMCID: PMC6758796
- DOI: 10.1523/JNEUROSCI.22-11-04428.2002
Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse
Abstract
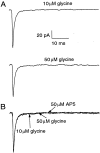
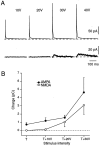
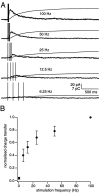
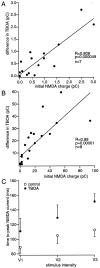
We have identified an excitatory synapse in cerebellar molecular layer interneurons at which the level of presynaptic activity determines the receptor type involved in the postsynaptic response. When small numbers of parallel fibers are activated, EPSCs are mediated solely by AMPA receptors (AMPARs), despite our finding that NMDA receptors (NMDARs) are present in the dendrites of these cells. The EPSC kinetics are fast (tau decay = 0.82 +/- 0.05 msec at room temperature), consistent with the role these interneurons are thought to play in precisely timed inhibitory control of Purkinje cells. NMDARs are activated only when glutamate release is increased either by facilitation with brief high-frequency trains or by recruiting more presynaptic fibers with higher stimulus intensities. Under these conditions, EPSCs consist of a fast-rising AMPAR-mediated current followed by a slow component mediated by both NMDARs and AMPARs. Inhibitors of glutamate transport increase the amplitude and prolong the time course of the compound EPSCs. In contrast, the properties of fast AMPAR EPSCs resulting from the activation of few inputs remain unchanged when glutamate uptake is blocked. Our results suggest that, at these synapses, the postsynaptic density contains AMPARs alone. It is only when transmitter release is high enough for glutamate to diffuse to the extrasynaptic space and to reach concentrations sufficient to activate extrasynaptic receptors that NMDARs are involved in the postsynaptic response. We suggest that such a spatial separation of receptor types may provide a mechanism for rapid changes in EPSC properties, depending on the amount of synaptic activity.
Figures

Similar articles
-
Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina.J Neurosci. 2002 Mar 15;22(6):2165-73. doi: 10.1523/JNEUROSCI.22-06-02165.2002. J Neurosci. 2002. PMID: 11896156 Free PMC article.
-
The speeding of EPSC kinetics during maturation of a central synapse.Eur J Neurosci. 2002 Mar;15(5):785-97. doi: 10.1046/j.1460-9568.2002.01910.x. Eur J Neurosci. 2002. PMID: 11906520
-
Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse.J Neurosci. 2000 Jun 15;20(12):4423-34. doi: 10.1523/JNEUROSCI.20-12-04423.2000. J Neurosci. 2000. PMID: 10844011 Free PMC article.
-
Developmental plasticity of NMDA receptors at the calyx of Held synapse.Neuropharmacology. 2021 Sep 15;196:108697. doi: 10.1016/j.neuropharm.2021.108697. Epub 2021 Jul 6. Neuropharmacology. 2021. PMID: 34242682 Review.
-
Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation.Sci Signal. 2019 Jan 1;12(562):eaar6889. doi: 10.1126/scisignal.aar6889. Sci Signal. 2019. PMID: 30600260 Free PMC article. Review.
Cited by
-
Synaptic metaplasticity through NMDA receptor lateral diffusion.J Neurosci. 2008 Mar 19;28(12):3060-70. doi: 10.1523/JNEUROSCI.5450-07.2008. J Neurosci. 2008. Retraction in: J Neurosci. 2011 Jun 22;31(25):9441. PMID: 18354009 Free PMC article. Retracted.
-
High Salt Intake Recruits Tonic Activation of NR2D Subunit-Containing Extrasynaptic NMDARs in Vasopressin Neurons.J Neurosci. 2021 Feb 10;41(6):1145-1156. doi: 10.1523/JNEUROSCI.1742-20.2020. Epub 2020 Dec 10. J Neurosci. 2021. PMID: 33303677 Free PMC article.
-
Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina.J Comp Neurol. 2006 Oct 20;498(6):810-20. doi: 10.1002/cne.21089. J Comp Neurol. 2006. PMID: 16927255 Free PMC article.
-
Chronic intermittent hypoxia affects integration of sensory input by neurons in the nucleus tractus solitarii.Respir Physiol Neurobiol. 2010 Nov 30;174(1-2):29-36. doi: 10.1016/j.resp.2010.04.015. Epub 2010 Apr 21. Respir Physiol Neurobiol. 2010. PMID: 20416405 Free PMC article.
-
Evaluation of glutamate concentration transient in the synaptic cleft of the rat calyx of Held.J Physiol. 2013 Jan 1;591(1):219-39. doi: 10.1113/jphysiol.2012.241398. Epub 2012 Oct 15. J Physiol. 2013. PMID: 23070699 Free PMC article.
References
-
- Asztely F, Erdemli G, Kullman DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. - PubMed
-
- Barbour B, Häusser M. Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 1997;20:377–384. - PubMed
-
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. - PubMed
-
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
