Regional calcium regulation within cultured Drosophila neurons: effects of altered cAMP metabolism by the learning mutations dunce and rutabaga
- PMID: 12040051
- PMCID: PMC6758795
- DOI: 10.1523/JNEUROSCI.22-11-04437.2002
Regional calcium regulation within cultured Drosophila neurons: effects of altered cAMP metabolism by the learning mutations dunce and rutabaga
Abstract
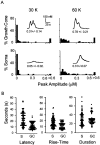
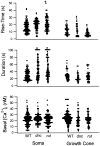
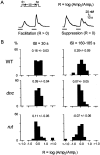
The dunce (dnc) and rutabaga (rut) mutations of Drosophila affect a cAMP-dependent phosphodiesterase and a Ca(2+)/CaM-regulated adenylyl cyclase, respectively. These mutations cause deficiencies in several learning paradigms and alter synaptic transmission, growth cone motility, and action potential generation. The cellular phenotypes either are Ca(2+) dependent (neurotransmission and motility) or mediate a Ca(2+) rise (action potential generation). However, interrelations among these defects have not been addressed. We have established conditions for fura-2 imaging of Ca(2+) dynamics in the "giant" neuron culture system of Drosophila. Using high K(+) depolarization of isolated neurons, we observed a larger, faster, and more dynamic response from the growth cone than the cell body. This Ca(2+) increase depended on an influx through Ca(2+) channels and was suppressed by the Na(+) channel blocker TTX. Altered cAMP metabolism by the dnc and rut mutations reduced response amplitude in the growth cone while prolonging the response within the soma. The enhanced spatial resolution of these larger cells allowed us to analyze Ca(2+) regulation within distinct domains of mutant growth cones. Modulation by a previous conditioning stimulus was altered in terms of response amplitude and waveform complexity. Furthermore, rut disrupted the distinction in Ca(2+) responses observed between the periphery and central domain of growth cones with motile filopodia.
Figures






Similar articles
-
Sub-cellular Ca2+ dynamics affected by voltage- and Ca2+-gated K+ channels: Regulation of the soma-growth cone disparity and the quiescent state in Drosophila neurons.Neuroscience. 2006 Oct 27;142(3):629-44. doi: 10.1016/j.neuroscience.2006.06.051. Epub 2006 Aug 17. Neuroscience. 2006. PMID: 16919393
-
Modulation of dihydropyridine-sensitive calcium channels in Drosophila by a cAMP-mediated pathway.J Neurobiol. 1999 Jun 15;39(4):491-500. doi: 10.1002/(sici)1097-4695(19990615)39:4<491::aid-neu3>3.0.co;2-6. J Neurobiol. 1999. PMID: 10380071
-
Cyclic adenosine monophosphate metabolism in synaptic growth, strength, and precision: neural and behavioral phenotype-specific counterbalancing effects between dnc phosphodiesterase and rut adenylyl cyclase mutations.J Neurogenet. 2012 Mar;26(1):64-81. doi: 10.3109/01677063.2011.652752. Epub 2012 Mar 1. J Neurogenet. 2012. PMID: 22380612 Free PMC article.
-
Differential modulation of potassium currents by cAMP and its long-term and short-term effects: dunce and rutabaga mutants of Drosophila.J Neurogenet. 1993 Aug;9(1):15-27. doi: 10.3109/01677069309167273. J Neurogenet. 1993. PMID: 8295075
-
Genetic dissection of functional contributions of specific potassium channel subunits in habituation of an escape circuit in Drosophila.J Neurosci. 1998 Mar 15;18(6):2254-67. doi: 10.1523/JNEUROSCI.18-06-02254.1998. J Neurosci. 1998. PMID: 9482810 Free PMC article. Review.
Cited by
-
Temperature-dependent developmental plasticity of Drosophila neurons: cell-autonomous roles of membrane excitability, Ca2+ influx, and cAMP signaling.J Neurosci. 2007 Nov 14;27(46):12611-22. doi: 10.1523/JNEUROSCI.2179-07.2007. J Neurosci. 2007. PMID: 18003840 Free PMC article.
-
Cyclic nucleotide signaling is required during synaptic refinement at the Drosophila neuromuscular junction.Dev Neurobiol. 2017 Jan;77(1):39-60. doi: 10.1002/dneu.22407. Epub 2016 Jun 20. Dev Neurobiol. 2017. PMID: 27281494 Free PMC article.
-
Drosophila cacophony channels: a major mediator of neuronal Ca2+ currents and a trigger for K+ channel homeostatic regulation.J Neurosci. 2007 Jan 31;27(5):1072-81. doi: 10.1523/JNEUROSCI.4746-06.2007. J Neurosci. 2007. PMID: 17267561 Free PMC article.
-
The role of cAMP in synaptic homeostasis in response to environmental temperature challenges and hyperexcitability mutations.Front Cell Neurosci. 2015 Feb 2;9:10. doi: 10.3389/fncel.2015.00010. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25698925 Free PMC article.
-
D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila.J Neurosci. 2007 Jul 18;27(29):7640-7. doi: 10.1523/JNEUROSCI.1167-07.2007. J Neurosci. 2007. PMID: 17634358 Free PMC article.
References
-
- Aceves-Pina EO, Booker R, Duerr JS, Livingstone MS, Quinn WG, Smith RF, Sziber PP, Tempel BL, Tully T. Learning and memory in Drosophila, studied with mutants. Cold Spring Harb Symp Quant Biol. 1983;48:831–839. - PubMed
-
- Alshuaib W, Byerly L. Modulation of membrane currents by cAMP in cleavage-arrested Drosophila neurons. J Exp Biol. 1996;199:537–548. - PubMed
-
- Azhderian EM, Hefner D, Lin CH, Kaczmarek LK, Forscher P. Cyclic AMP modulates fast axonal transport in Aplysia bag cell neurons by increasing the probability of single organelle movement. Neuron. 1994;12:1223–1233. - PubMed
-
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci. 2000;1:11–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
