Developmental regulation of small-conductance Ca2+-activated K+ channel expression and function in rat Purkinje neurons
- PMID: 12040053
- PMCID: PMC6758803
- DOI: 10.1523/JNEUROSCI.22-11-04456.2002
Developmental regulation of small-conductance Ca2+-activated K+ channel expression and function in rat Purkinje neurons
Abstract
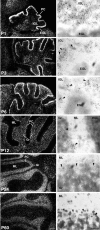
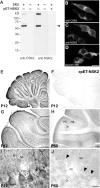
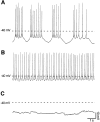
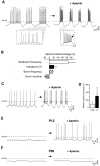
Calcium transients play an important role in the early and later phases of differentiation and maturation of single neurons and neuronal networks. Small-conductance calcium-activated potassium channels of the SK type modulate membrane excitability and are important determinants of the firing properties of central neurons. Increases in the intracellular calcium concentration activate SK channels, leading to a hyperpolarization of the membrane potential, which in turn reduces the calcium inflow into the cell. This feedback mechanism is ideally suited to regulate the spatiotemporal occurrence of calcium transients. However, the role of SK channels in neuronal development has not been addressed so far. We have concentrated on the ontogenesis and function of SK channels in the developing rat cerebellum, focusing particularly on Purkinje neurons. Electrophysiological recordings combined with specific pharmacological tools have revealed for the first time the presence of an afterhyperpolarizing current (I(AHP)) in immature Purkinje cells in rat cerebellar slices. The channel subunits underlying this current were identified as SK2 and localized by in situ hybridization and subunit-specific antibodies. Their expression level was shown to be high at birth and subsequently to decline during the first 3 weeks of postnatal life, both at the mRNA and protein levels. This developmental regulation was tightly correlated with the expression of I(AHP) and the prominent role of SK2 channels in shaping the spontaneous firing pattern in young, but not in adult, Purkinje neurons. These results provide the first evidence of the developmental regulation and function of SK channels in central neurons.
Figures


References
-
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–1709. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145:399–463. - PubMed
-
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure, and functions. CRC; Boca Raton, FL: 1997.
-
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. - PubMed
-
- Crepel F. Maturation of the cerebellar Purkinje cells. I. Postnatal evolution of the Purkinje cell spontaneous firing in the rat. Exp Brain Res. 1972;14:463–471.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
