Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress
- PMID: 12040062
- PMCID: PMC6758774
- DOI: 10.1523/JNEUROSCI.22-11-04550.2002
Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress
Abstract
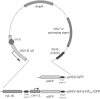
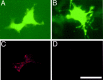
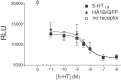
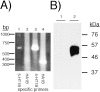
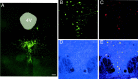
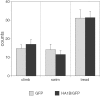
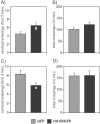
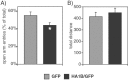
5-HT(1B) autoreceptors have been implicated in animal models of stress and are regulated selectively by serotonin-selective reuptake inhibitors such as fluoxetine. These terminal autoreceptors regulate serotonin release from dorsal raphe nucleus (DRN) projections throughout rat forebrain. However, it has not been previously possible to manipulate 5-HT(1B) autoreceptor activity selectively without also changing 5-HT(1B) activity in other neurons mediating different behavioral responses. Therefore, we have developed a viral-mediated gene transfer strategy to express hemagglutinin-tagged 5-HT(1B) and manipulate these autoreceptors in DRN. Green fluorescent protein (GFP) was coexpressed from a separate transcriptional unit on the same amplicon to assist in monitoring infection and expression. We confirmed the expression and biological activity of both transgenic proteins in vitro. When injected directly into DRN using stereotaxic procedure, HA-5-HT(1B) receptors were expressed in serotonergic neurons and translocated to the forebrain. The effect of DRN expression of HA-5-HT(1B) on stress-induced behaviors was compared with control rats that received GFP-only amplicons. There was no change in immobility in the forced swim test. However, HA-5-HT(1B) expression significantly reduced entrances into the central region of an open-field arena after water-restraint stress without altering overall locomotor activity, but not in the absence of stress exposure. HA-5-HT(1B) expression also reduced entries into the open arms of the elevated plus maze after water restraint. Because these tests are sensitive to increases in anxiety-like behavior, our results suggest that overactivity of 5-HT(1B) autoreceptors in DRN neurons may be an important mediator of pathological responses to stressful events.
Figures


References
-
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. - PubMed
-
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. - PubMed
-
- Anthony JP, Sexton TJ, Neumaier JF. Antidepressant induced regulation of 5-HT1B mRNA in rat dorsal raphe nucleus reverses rapidly following drug discontinuation. J Neurosci Res. 2000;61:82–87. - PubMed
-
- Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. - PubMed
-
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
