Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease
- PMID: 12040070
- PMCID: PMC6758785
- DOI: 10.1523/JNEUROSCI.22-11-04639.2002
Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease
Abstract
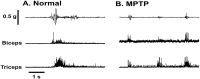
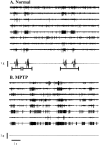
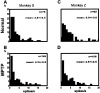
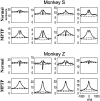
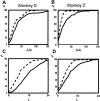
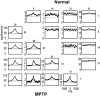
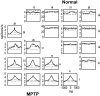
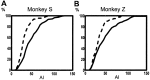
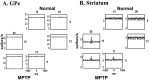
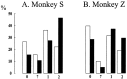
Primary motor cortex (MI) neurons discharge vigorously during voluntary movement. A cardinal symptom of Parkinson's disease (PD) is poverty of movement (akinesia). Current models of PD thus hypothesize that increased inhibitory pallidal output reduces firing rates in frontal cortex, including MI, resulting in akinesia and muscle rigidity. We recorded the simultaneous spontaneous discharge of several neurons in the arm-related area of MI of two monkeys and in the globus pallidus (GP) of one of the two. Accelerometers were fastened to the forelimbs to detect movement, and surface electromyograms were recorded from the contralateral arm of one monkey. The recordings were conducted before and after systemic treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rendering the animals severely akinetic and rigid with little or no tremor. The mean spontaneous MI rates during periods of immobility (four to five spikes/sec) did not change after MPTP; however, in this parkinsonian state, MI neurons discharged in long bursts (sometimes >2 sec long). These bursts were synchronized across many cells but failed to elicit detectable movement, indicating that even robust synchronous MI discharge need not result in movement. These synchronized population bursts were absent from the GP and were on a larger timescale than oscillatory synchrony found in the GP of tremulous MPTP primates, suggesting that MI parkinsonian synchrony arises independently of basal ganglia dynamics. After MPTP, MI neurons responded more vigorously and with less specificity to passive limb movement. Abnormal MI firing patterns and synchronization, rather than reduced firing rates, may underlie PD akinesia and persistent muscle rigidity.
Figures



Similar articles
-
Computational physiology of the basal ganglia in Parkinson's disease.Prog Brain Res. 2010;183:259-73. doi: 10.1016/S0079-6123(10)83013-4. Prog Brain Res. 2010. PMID: 20696324
-
Low-pass filter properties of basal ganglia cortical muscle loops in the normal and MPTP primate model of parkinsonism.J Neurosci. 2008 Jan 16;28(3):633-49. doi: 10.1523/JNEUROSCI.3388-07.2008. J Neurosci. 2008. PMID: 18199764 Free PMC article.
-
The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism.J Neurophysiol. 1994 Aug;72(2):507-20. doi: 10.1152/jn.1994.72.2.507. J Neurophysiol. 1994. PMID: 7983515
-
Pathophysiology of parkinsonian motor abnormalities.Adv Neurol. 1993;60:53-61. Adv Neurol. 1993. PMID: 8420185 Review. No abstract available.
-
The actions of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in animals as a model of Parkinson's disease.J Neural Transm Suppl. 1986;20:11-39. J Neural Transm Suppl. 1986. PMID: 3091760 Review.
Cited by
-
Decreased cellular excitability of pyramidal tract neurons in primary motor cortex leads to paradoxically increased network activity in simulated parkinsonian motor cortex.bioRxiv [Preprint]. 2024 Jun 23:2024.05.23.595566. doi: 10.1101/2024.05.23.595566. bioRxiv. 2024. Update in: NPJ Parkinsons Dis. 2025 Aug 5;11(1):230. doi: 10.1038/s41531-025-01070-4. PMID: 38948850 Free PMC article. Updated. Preprint.
-
Decoupling of interacting neuronal populations by time-shifted stimulation through spike-timing-dependent plasticity.PLoS Comput Biol. 2023 Feb 1;19(2):e1010853. doi: 10.1371/journal.pcbi.1010853. eCollection 2023 Feb. PLoS Comput Biol. 2023. PMID: 36724144 Free PMC article.
-
Rapid development of strong, persistent, spatiotemporally extensive cortical synchrony and underlying oscillations following acute MCA focal ischemia.Sci Rep. 2020 Dec 8;10(1):21441. doi: 10.1038/s41598-020-78179-4. Sci Rep. 2020. PMID: 33293620 Free PMC article.
-
Motor learning in animal models of Parkinson's disease: Aberrant synaptic plasticity in the motor cortex.Mov Disord. 2017 Apr;32(4):487-497. doi: 10.1002/mds.26938. Epub 2017 Mar 25. Mov Disord. 2017. PMID: 28343366 Free PMC article. Review.
-
Physiological identification of the human pedunculopontine nucleus.J Neurol Neurosurg Psychiatry. 2010 Jan;81(1):80-6. doi: 10.1136/jnnp.2009.179069. Epub 2009 Oct 14. J Neurol Neurosurg Psychiatry. 2010. PMID: 19828478 Free PMC article.
References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
-
- Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci. 2002;25:27–31. - PubMed
-
- Asanuma H. The motor cortex. Raven; New York: 1989.
-
- Bar-Gad I, Ritov Y, Vaadia E, Bergman H. Failure in identification of overlapping spikes from multiple neuron activity causes artificial correlations. J Neurosci Methods. 2001;107:1–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
