PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia
- PMID: 12040081
- PMCID: PMC6758778
- DOI: 10.1523/JNEUROSCI.22-11-04740.2002
PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia
Abstract
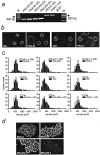
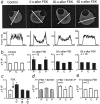
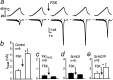
Inflammatory mediators not only activate "pain-"sensing neurons, the nociceptors, to trigger acute pain sensations, more important, they increase nociceptor responsiveness to produce inflammatory hyperalgesia. For example, prostaglandins activate G(s)-protein-coupled receptors and initiate cAMP- and protein kinase A (PKA)-mediated processes. We demonstrate for the first time at the cellular level that heat-activated ionic currents were potentiated after exposure to the cAMP activator forskolin in rat nociceptive neurons. The potentiation was prevented in the presence of the selective PKA inhibitor PKI(14-22), suggesting PKA-mediated phosphorylation of the heat transducer protein. PKA regulatory subunits were found in close vicinity to the plasma membrane in these neurons, and PKA catalytic subunits only translocated to the cell periphery when activated. The translocation and the current potentiation were abolished in the presence of an A-kinase anchoring protein (AKAP) inhibitor. Similar current changes after PKA activation were obtained from human embryonic kidney 293t cells transfected with the wild-type heat transducer protein vanilloid receptor 1 (VR-1). The forskolin-induced current potentiation was greatly reduced in cells transfected with VR-1 mutants carrying point mutations at the predicted PKA phosphorylation sites. The heat transducer VR-1 is therefore suggested as the molecular target of PKA phosphorylation, and potentiation of current responses to heat depends on phosphorylation at predicted PKA consensus sites. Thus, the PKA/AKAP/VR-1 module presents as the molecular correlate of G(s)-mediated inflammatory hyperalgesia.
Figures



Similar articles
-
Fast Ca2+-induced potentiation of heat-activated ionic currents requires cAMP/PKA signaling and functional AKAP anchoring.J Neurophysiol. 2003 May;89(5):2499-505. doi: 10.1152/jn.00713.2002. J Neurophysiol. 2003. PMID: 12740405
-
Relative roles of protein kinase A and protein kinase C in modulation of transient receptor potential vanilloid type 1 receptor responsiveness in rat sensory neurons in vitro and peripheral nociceptors in vivo.Neuroscience. 2006 Jun 30;140(2):645-57. doi: 10.1016/j.neuroscience.2006.02.035. Epub 2006 Mar 29. Neuroscience. 2006. PMID: 16564637
-
Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons.J Neurosci. 2008 May 7;28(19):4904-17. doi: 10.1523/JNEUROSCI.0233-08.2008. J Neurosci. 2008. PMID: 18463244 Free PMC article.
-
Acidosis Mediates the Switching of Gs-PKA and Gi-PKCε Dependence in Prolonged Hyperalgesia Induced by Inflammation.PLoS One. 2015 May 1;10(5):e0125022. doi: 10.1371/journal.pone.0125022. eCollection 2015. PLoS One. 2015. PMID: 25933021 Free PMC article.
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
Cited by
-
The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence.Channels (Austin). 2015;9(5):235-43. doi: 10.1080/19336950.2015.1069450. Epub 2015 Jul 15. Channels (Austin). 2015. PMID: 26176938 Free PMC article. Review.
-
Modulation of osmotic stress-induced TRPV1 expression rescues human iPSC-derived retinal ganglion cells through PKA.Stem Cell Res Ther. 2019 Sep 23;10(1):284. doi: 10.1186/s13287-019-1363-1. Stem Cell Res Ther. 2019. PMID: 31547874 Free PMC article.
-
Light-dependent phosphorylation of the drosophila transient receptor potential ion channel.J Biol Chem. 2010 May 7;285(19):14275-84. doi: 10.1074/jbc.M110.102053. Epub 2010 Mar 9. J Biol Chem. 2010. PMID: 20215118 Free PMC article.
-
TRPV1 antagonists attenuate antigen-provoked cough in ovalbumin sensitized guinea pigs.Cough. 2006 Dec 15;2:10. doi: 10.1186/1745-9974-2-10. Cough. 2006. PMID: 17173683 Free PMC article.
-
Scaffolding by A-kinase anchoring protein enhances functional coupling between adenylyl cyclase and TRPV1 channel.J Biol Chem. 2013 Feb 8;288(6):3929-37. doi: 10.1074/jbc.M112.428144. Epub 2012 Dec 21. J Biol Chem. 2013. PMID: 23264624 Free PMC article.
References
-
- Cannon SC, Strittmatter SM. Functional expression of sodium channel mutations identified in families with periodic paralysis. Proc Natl Acad Sci USA. 1993;90:5327–5331. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. - PubMed
-
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton P. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Biophys J. 1999;23:617–624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
