Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs
- PMID: 12040083
- PMCID: PMC6758812
- DOI: 10.1523/JNEUROSCI.22-11-04756.2002
Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs
Abstract
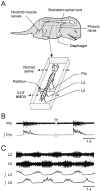
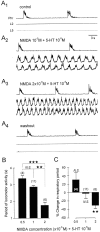
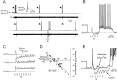
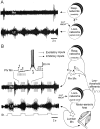
A 1:1 coordination between locomotor and respiratory movements has been described in various mammalian species during fast locomotion, and several mechanisms underlying such interactions have been proposed. Here we use an isolated brainstem-spinal cord preparation of the neonatal rat to determine the origin of this coupling, which could derive either from a direct interaction between the central locomotor- and respiratory-generating networks themselves or from an indirect influence via a peripheral mechanism. We demonstrate that during fictive locomotion induced by pharmacological activation of the lumbar locomotor generators, a concomitant increase in spontaneous respiratory rate occurs without any evident form of phase coupling. In contrast, respiratory motor activity can be fully entrained (1:1 coupling) over a range of periodic electrical stimulation applied to low-threshold sensory pathways originating from hindlimb muscles. Our results provide strong support for the existence of pathways between lumbar proprioceptive afferents, medullary respiratory networks, and phrenic motoneurons that could provide the basis of the locomotor-respiratory coupling in many animals. Thus a peripheral sensory system involved in a well defined rhythmic motor function can be responsible for the tight functional interaction between two otherwise independent motor behaviors.
Figures



References
-
- Ballion B, Morin D, Viala D. Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur J Neurosci. 2001;14:1727–1738. - PubMed
-
- Banzett RB, Mead J, Reid MB, Topulos GP. Locomotion in men has no appreciable mechanical effect on breathing. J Appl Physiol. 1992;72:1922–1926. - PubMed
-
- Bracci E, Beato M, Nistri A. Afferent inputs modulate the activity of a rhythmic burst generator in the rat disinhibited spinal cord in vitro. J Neurophysiol. 1997;77:3157–3167. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
