Osmotic stress signaling and osmoadaptation in yeasts
- PMID: 12040128
- PMCID: PMC120784
- DOI: 10.1128/MMBR.66.2.300-372.2002
Osmotic stress signaling and osmoadaptation in yeasts
Abstract
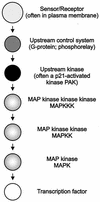
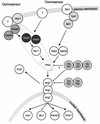
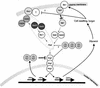
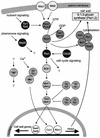
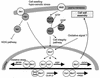
The ability to adapt to altered availability of free water is a fundamental property of living cells. The principles underlying osmoadaptation are well conserved. The yeast Saccharomyces cerevisiae is an excellent model system with which to study the molecular biology and physiology of osmoadaptation. Upon a shift to high osmolarity, yeast cells rapidly stimulate a mitogen-activated protein (MAP) kinase cascade, the high-osmolarity glycerol (HOG) pathway, which orchestrates part of the transcriptional response. The dynamic operation of the HOG pathway has been well studied, and similar osmosensing pathways exist in other eukaryotes. Protein kinase A, which seems to mediate a response to diverse stress conditions, is also involved in the transcriptional response program. Expression changes after a shift to high osmolarity aim at adjusting metabolism and the production of cellular protectants. Accumulation of the osmolyte glycerol, which is also controlled by altering transmembrane glycerol transport, is of central importance. Upon a shift from high to low osmolarity, yeast cells stimulate a different MAP kinase cascade, the cell integrity pathway. The transcriptional program upon hypo-osmotic shock seems to aim at adjusting cell surface properties. Rapid export of glycerol is an important event in adaptation to low osmolarity. Osmoadaptation, adjustment of cell surface properties, and the control of cell morphogenesis, growth, and proliferation are highly coordinated processes. The Skn7p response regulator may be involved in coordinating these events. An integrated understanding of osmoadaptation requires not only knowledge of the function of many uncharacterized genes but also further insight into the time line of events, their interdependence, their dynamics, and their spatial organization as well as the importance of subtle effects.
Figures





References
-
- Aguilera, J., and J. A. Prieto. 2001. The Saccharomyces cerevisiae aldose reductase is implied in the metabolism of methylglyoxal in response to stress conditions. Curr. Genet. 39:273-283. - PubMed
-
- Aiba, H., H. Yamada, R. Ohmiya, and T. Mizuno. 1995. The osmo-inducible gpd1+ gene is a target of the signaling pathway involving Wis1 MAP-kinase kinase in fission yeast. FEBS Lett. 376:199-201. - PubMed
-
- Akada, R., J. Yamamoto, and I. Yamashita. 1997. Screening and identification of yeast sequences that cause growth inhibition when overexpressed. Mol. Gen. Genet. 254:267-274. - PubMed
-
- Akhtar, N., A. K. Pahlman, K. Larsson, A. H. Corbett, and L. Adler. 2000. SGD1 encodes an essential nuclear protein of Saccharomyces cerevisiae that affects expression of the GPD1 gene for glycerol 3-phosphate dehydrogenase. FEBS Lett. 483:87-92. - PubMed
-
- Alberts, A. S., N. Bouquin, L. H. Johnston, and R. Treisman. 1998. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J. Biol. Chem. 273:8616-8622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

