Molecular aspects of acute inhibition of Na(+)-H(+) exchanger NHE3 by A(2)-adenosine receptor agonists
- PMID: 12042357
- PMCID: PMC2290320
- DOI: 10.1113/jphysiol.2001.013438
Molecular aspects of acute inhibition of Na(+)-H(+) exchanger NHE3 by A(2)-adenosine receptor agonists
Abstract
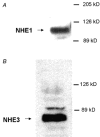
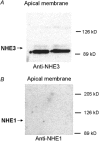
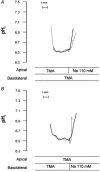
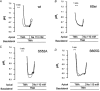
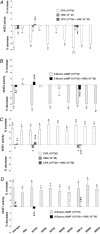
Adenosine regulates Na(+) homeostasis by its acute effects on renal Na(+) transport. We have shown in heterologously transfected A6/C1 cells (renal cell line from Xenopus laevis) that adenosine-induced natriuresis may be effected partly via A(2) adenosine receptor-mediated inactivation of the renal brush border membrane Na(+)-H(+) exchanger NHE3. In this study we utilized A6/C1 cells stably expressing wild-type as well as mutated forms of NHE3 to assess the molecular mechanism underlying A(2)-dependent control of NHE3 function. Cell surface biotinylation combined with immunoprecipitation revealed that NHE3 is targeted exclusively to the apical domain and that the endogenous Xenopus NHE is located entirely on the basolateral side of A6/C1 transfectants. Stimulation of A(2)-adenosine receptors located on the basolateral side for 15 min with CPA (N6-cyclopentyladenosine) acutely decreased NHE3 activity (microspectrofluorimety). This effect was mimicked by 8-bromo-cAMP and entirely blocked by pharmacological inhibition of PKA (with H89) or singular substitution of two PKA target sites (serine 552 and serine 605) on NHE3. Downregulation of NHE3 activity by CPA was attributable to a reduction of NHE3 intrinsic transport activity without change in surface NHE3 protein at 15 min. At 30 min, the decrease in transport activity was associated with a decrease in apical membrane NHE3 antigen. In conclusion, two highly conserved target serine sites on NHE3 determine NHE3 modulation upon A(2)-receptor activation and NHE3 inactivation by adenosine proceeds via two phases with distinct mechanisms.
Figures

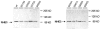

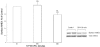
Similar articles
-
Adenosine inhibits the transfected Na+-H+ exchanger NHE3 in Xenopus laevis renal epithelial cells (A6/C1).J Physiol. 1999 Mar 15;515 ( Pt 3)(Pt 3):829-42. doi: 10.1111/j.1469-7793.1999.829ab.x. J Physiol. 1999. PMID: 10066908 Free PMC article.
-
Bimodal acute effects of A1 adenosine receptor activation on Na+/H+ exchanger 3 in opossum kidney cells.J Am Soc Nephrol. 2003 Jul;14(7):1720-30. doi: 10.1097/01.asn.0000072743.97583.db. J Am Soc Nephrol. 2003. PMID: 12819231
-
Regulation of the transfected Na+/H+-exchanger NHE3 in MDCK cells by vasotocin.Pflugers Arch. 1997 May;434(1):123-31. doi: 10.1007/s004240050372. Pflugers Arch. 1997. PMID: 9094265
-
Signal complex regulation of renal transport proteins: NHERF and regulation of NHE3 by PKA.Am J Physiol Renal Physiol. 2000 Sep;279(3):F393-9. doi: 10.1152/ajprenal.2000.279.3.F393. Am J Physiol Renal Physiol. 2000. PMID: 10966919 Review.
-
Short-term regulation of NHE3 by EGF and protein kinase C but not protein kinase A involves vesicle trafficking in epithelial cells and fibroblasts.Ann N Y Acad Sci. 2000;915:30-42. doi: 10.1111/j.1749-6632.2000.tb05221.x. Ann N Y Acad Sci. 2000. PMID: 11193592 Review.
Cited by
-
Adenosine stimulates the basolateral 50 pS K+ channel in renal proximal tubule via adenosine-A1 receptor.Front Physiol. 2023 Aug 28;14:1242975. doi: 10.3389/fphys.2023.1242975. eCollection 2023. Front Physiol. 2023. PMID: 37700760 Free PMC article.
-
Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo.J Clin Invest. 2006 Oct;116(10):2682-94. doi: 10.1172/JCI29218. J Clin Invest. 2006. PMID: 17016558 Free PMC article.
-
The reduction of Na/H exchanger-3 protein and transcript expression in acute ischemia-reperfusion injury is mediated by extractable tissue factor(s).Kidney Int. 2011 Oct;80(8):822-831. doi: 10.1038/ki.2011.229. Epub 2011 Aug 3. Kidney Int. 2011. PMID: 21814178 Free PMC article.
-
Cyclic AMP-mediated endocytosis of intestinal epithelial NHE3 requires binding to synaptotagmin 1.Am J Physiol Gastrointest Liver Physiol. 2010 Feb;298(2):G203-11. doi: 10.1152/ajpgi.00379.2009. Epub 2009 Nov 19. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 19926819 Free PMC article.
-
Purinergic regulation of epithelial transport.J Physiol. 2004 Mar 1;555(Pt 2):311-21. doi: 10.1113/jphysiol.2003.056697. Epub 2003 Dec 23. J Physiol. 2004. PMID: 14694149 Free PMC article. Review.
References
-
- Amemiya M, Loffing J, Lötscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney International. 1995;48:1206–1215. - PubMed
-
- Aronson PS. Role of ion exchangers in mediating NaCl transport in the proximal tubule. Kidney International. 1996;49:1665–1670. - PubMed
-
- Beach RE, Good DW. Effects of adenosine on ion transport in rat medullary thick ascending limb. American Journal of Physiology. 1992;263:F482–487. - PubMed
-
- Chambrey R, Warnock DG, Podevin R-A, Bruneval P, Mandet C, Bélair M-F, Bariéty J, Paillard M. Immunolocalization of the Na+/H+ exchanger isoform NHE2 in rat kidney. American Journal of Physiology. 1998;275:F379–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

