Short-term synaptic plasticity, simulation of nerve terminal dynamics, and the effects of protein kinase C activation in rat hippocampus
- PMID: 12042358
- PMCID: PMC2290341
- DOI: 10.1113/jphysiol.2001.015842
Short-term synaptic plasticity, simulation of nerve terminal dynamics, and the effects of protein kinase C activation in rat hippocampus
Abstract
Phorbol esters are hypothesised to produce a protein kinase C (PKC)-dependent increase in the probability of transmitter release via two mechanisms: facilitation of vesicle fusion or increases in synaptic vesicle number and replenishment. We used a combination of electrophysiology and computer simulation to distinguish these possibilities. We constructed a stochastic model of the presynaptic contacts between a pair of hippocampal pyramidal cells that used biologically realistic processes and was constrained by electrophysiological data. The model reproduced faithfully several forms of short-term synaptic plasticity, including short-term synaptic depression (STD), and allowed us to manipulate several experimentally inaccessible processes. Simulation of an increase in the size of the readily releasable vesicle pool and the time of vesicle replenishment decreased STD, whereas simulation of a facilitation of vesicle fusion downstream of Ca(2+) influx enhanced STD. Because activation of protein kinase C with phorbol ester enhanced STD of EPSCs in rat hippocampal slice cultures, we conclude that an increase in the sensitivity of the release process for Ca(2+) underlies the potentiation of neurotransmitter release by PKC.
Figures
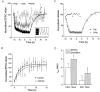
 , n = 4), 3 Hz (○, n = 5) and 10 Hz (•, n = 12) stimulation for 15 s. Lines show the exponential fits to 3 Hz (dashed) and 10 Hz (continuous) recovery data points. Inset: stimulation protocol for assessing recovery from STD. After tetanic stimulation, stimuli were delivered at 0.25 Hz, with the first stimulus offset by 1 s over 4 trials. B, recovery from STD (10 Hz, 15 s) before and after application of the A1 antagonist DPCPX. Lines show the exponential fits to vehicle (continuous) and DPCPX (dashed) data points. C, simulations of tetanic stimulation using a mechanism in which vesicle replenishment required 1 s, but was capacity limited (see text). Depression and recovery in response to 1 Hz (•) and 10 Hz (○) stimulation was consistent with EPSC data. The continuous line indicates exponential fit of recovery data. D, time constants of exponentials for recovery from simulated release (hatched bars) and EPSCs (open bars) induced with 3 and 10 Hz stimulation. *P < 0.01 vs. 10 Hz stimulation. Recovery of both EPSCs and simulated release is faster after 3 Hz stimulation than after 10 Hz stimulation.
, n = 4), 3 Hz (○, n = 5) and 10 Hz (•, n = 12) stimulation for 15 s. Lines show the exponential fits to 3 Hz (dashed) and 10 Hz (continuous) recovery data points. Inset: stimulation protocol for assessing recovery from STD. After tetanic stimulation, stimuli were delivered at 0.25 Hz, with the first stimulus offset by 1 s over 4 trials. B, recovery from STD (10 Hz, 15 s) before and after application of the A1 antagonist DPCPX. Lines show the exponential fits to vehicle (continuous) and DPCPX (dashed) data points. C, simulations of tetanic stimulation using a mechanism in which vesicle replenishment required 1 s, but was capacity limited (see text). Depression and recovery in response to 1 Hz (•) and 10 Hz (○) stimulation was consistent with EPSC data. The continuous line indicates exponential fit of recovery data. D, time constants of exponentials for recovery from simulated release (hatched bars) and EPSCs (open bars) induced with 3 and 10 Hz stimulation. *P < 0.01 vs. 10 Hz stimulation. Recovery of both EPSCs and simulated release is faster after 3 Hz stimulation than after 10 Hz stimulation.






References
-
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N. Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. - PubMed
-
- Capogna M, Fankhauser C, Gagliardini V, Gähwiler BH, Thompson SM. Excitatory synaptic transmission and its modulation by PKC is unchanged in the hippocampus of GAP-43-deficient mice. European Journal of Neuroscience. 1999;11:433–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

