Topographic reorganization in area 18 of adult cats following circumscribed monocular retinal lesions in adolescence
- PMID: 12042364
- PMCID: PMC2290339
- DOI: 10.1113/jphysiol.2001.016212
Topographic reorganization in area 18 of adult cats following circumscribed monocular retinal lesions in adolescence
Abstract
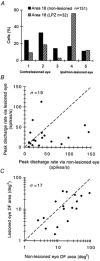
Circumscribed laser lesions were made in the nasal retinae of one eye in adolescent cats. Ten to sixteen months later, about 80 % of single neurones recorded in the lesion projection zone (LPZ) of contralateral area 18 (parastriate cortex, area V2) were binocular but when stimulated via the lesioned eye had ectopic discharge fields (displaced to normal retina in the vicinity of the lesion). Although the clear majority of binocular cells recorded from the LPZ responded with higher peak discharge rates to stimuli presented via the non-lesioned eye, the orientation and direction selectivities as well as preferred and upper cut-off velocities for stimuli presented through either eye were very similar. Furthermore, the sizes of the ectopic discharge fields of binocular cells recorded from the LPZ were not significantly different from those of their counterparts plotted via the non-lesioned eye. Thus, monocular retinal lesions performed in adolescent cats induce topographic reorganization in the LPZ of area 18. Although a similar reorganization occurs in area 17 (striate cortex, area V1) of cats in which monocular retinal lesions were made either in adulthood or adolescence, in view of the very different velocity response profiles of ectopic discharge fields in areas 17 and those in area 18, it appears that ectopic discharge fields in area 17 are largely independent of excitatory feedback input from area 18.
Figures

Similar articles
-
Plasticity in adult cat visual cortex (area 17) following circumscribed monocular lesions of all retinal layers.J Physiol. 2000 Apr 15;524 Pt 2(Pt 2):587-602. doi: 10.1111/j.1469-7793.2000.t01-1-00587.x. J Physiol. 2000. PMID: 10767137 Free PMC article.
-
Visuotopic reorganization in the primary visual cortex of adult cats following monocular and binocular retinal lesions.Cereb Cortex. 1996 May-Jun;6(3):388-405. doi: 10.1093/cercor/6.3.388. Cereb Cortex. 1996. PMID: 8670666
-
Laminar differences in plasticity in area 17 following retinal lesions in kittens or adult cats.Eur J Neurosci. 2003 Jun;17(11):2351-68. doi: 10.1046/j.1460-9568.2003.02674.x. Eur J Neurosci. 2003. PMID: 12814367
-
Cortical plasticity revealed by circumscribed retinal lesions or artificial scotomas.Prog Brain Res. 2001;134:217-46. doi: 10.1016/s0079-6123(01)34016-5. Prog Brain Res. 2001. PMID: 11702546 Review.
-
Binocular response modulation in the lateral geniculate nucleus.J Comp Neurol. 2019 Feb 15;527(3):522-534. doi: 10.1002/cne.24417. Epub 2018 Mar 9. J Comp Neurol. 2019. PMID: 29473163 Free PMC article. Review.
Cited by
-
The second face of blindness: processing speed deficits in the intact visual field after pre- and post-chiasmatic lesions.PLoS One. 2013 May 8;8(5):e63700. doi: 10.1371/journal.pone.0063700. Print 2013. PLoS One. 2013. PMID: 23667657 Free PMC article.
-
Plasticity Beyond V1: Reinforcement of Motion Perception upon Binocular Central Retinal Lesions in Adulthood.J Neurosci. 2017 Sep 13;37(37):8989-8999. doi: 10.1523/JNEUROSCI.1231-17.2017. Epub 2017 Aug 16. J Neurosci. 2017. PMID: 28821647 Free PMC article.
-
Homeostatic plasticity in human extrastriate cortex following a simulated peripheral scotoma.Exp Brain Res. 2017 Nov;235(11):3391-3401. doi: 10.1007/s00221-017-5042-0. Epub 2017 Aug 18. Exp Brain Res. 2017. PMID: 28821922
References
-
- Albus K. A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. I. The precision of the topography. Experimental Brain Research. 1975;24:159–179. - PubMed
-
- Alonso JM, Cudeiro J, Pérez R, Gonzalez F, Acuña C. Influence of layer V of area 18 of the cat visual cortex on responses of cells in layer V of area 17 to stimuli of high velocity. Experimental Brain Research. 1993a;93:363–366. - PubMed
-
- Alonso JM, Cudeiro J, Pérez R, Gonzalez F, Acuña C. Orientational influences of layer V of visual area 18 upon cells in layer V of area 17 in the cat cortex. Experimental Brain Research. 1993b;96:212–220. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

