Lipopolysaccharide endotoxins
- PMID: 12045108
- PMCID: PMC2569852
- DOI: 10.1146/annurev.biochem.71.110601.135414
Lipopolysaccharide endotoxins
Abstract
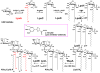
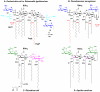
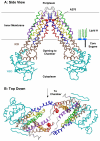
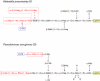
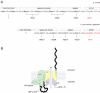
Bacterial lipopolysaccharides (LPS) typically consist of a hydrophobic domain known as lipid A (or endotoxin), a nonrepeating "core" oligosaccharide, and a distal polysaccharide (or O-antigen). Recent genomic data have facilitated study of LPS assembly in diverse Gram-negative bacteria, many of which are human or plant pathogens, and have established the importance of lateral gene transfer in generating structural diversity of O-antigens. Many enzymes of lipid A biosynthesis like LpxC have been validated as targets for development of new antibiotics. Key genes for lipid A biosynthesis have unexpectedly also been found in higher plants, indicating that eukaryotic lipid A-like molecules may exist. Most significant has been the identification of the plasma membrane protein TLR4 as the lipid A signaling receptor of animal cells. TLR4 belongs to a family of innate immunity receptors that possess a large extracellular domain of leucine-rich repeats, a single trans-membrane segment, and a smaller cytoplasmic signaling region that engages the adaptor protein MyD88. The expanding knowledge of TLR4 specificity and its downstream signaling pathways should provide new opportunities for blocking inflammation associated with infection.
Figures








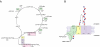

References
-
- Raetz CRH. Annual Reviews in Biochemistry. 1990;59:129–70. - PubMed
-
- Raetz CRH. Escherichia coli and Salmonella. In: Niedhardt FC, editor. Cellular and Molecular Biology. American Society for Microbiology; Washington, D.C.: 1996. pp. 1035–63.
-
- Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, et al. FASEB Journal. 1994;8:217–25. - PubMed
-
- Zähringer U, Lindner B, Rietschel ET. In: Endotoxin in Health and Disease. Brade H, Opal SM, Vogel SN, Morrison DC, editors. Marcel Dekker, Inc.; New York: 1999. pp. 93–114.
-
- Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in Health and Disease. Marcel Dekker, Inc.; New York: 1999. p. 950.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

