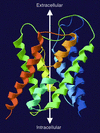
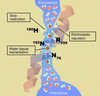
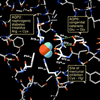
Aquaporin water channels: atomic structure molecular dynamics meet clinical medicine
- PMID: 12045251
- PMCID: PMC151002
- DOI: 10.1172/JCI15851
Aquaporin water channels: atomic structure molecular dynamics meet clinical medicine
Figures
References
-
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. - PubMed
-
- Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–458. - PubMed
-
- Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269:14648–14654. - PubMed
-
- Murata K, et al. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

