Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair
- PMID: 12045254
- PMCID: PMC151001
- DOI: 10.1172/JCI15681
Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair
Erratum in
- J Clin Invest 2002 Oct;110(8):1211
Abstract
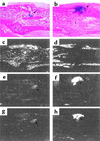
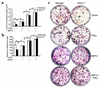
Preclinical and clinical studies suggest a possible role for cyclooxygenases in bone repair and create concerns about the use of nonsteroidal antiinflammatory drugs in patients with skeletal injury. We utilized wild-type, COX-1(-/-), and COX-2(-/-) mice to demonstrate that COX-2 plays an essential role in both endochondral and intramembranous bone formation during skeletal repair. The healing of stabilized tibia fractures was significantly delayed in COX-2(-/-) mice compared with COX-1(-/-) and wild-type controls. The histology was characterized by a persistence of undifferentiated mesenchyme and a marked reduction in osteoblastogenesis that resulted in a high incidence of fibrous nonunion in the COX-2(-/-) mice. Similarly, intramembranous bone formation on the calvaria was reduced 60% in COX-2(-/-) mice following in vivo injection of FGF-1 compared with either COX-1(-/-) or wild-type mice. To elucidate the mechanism involved in reduced bone formation, osteoblastogenesis was studied in bone marrow stromal cell cultures obtained from COX-2(-/-) and wild-type mice. Bone nodule formation was reduced 50% in COX-2(-/-) mice. The defect in osteogenesis was completely rescued by addition of prostaglandin E2 (PGE(2)) to the cultures. In the presence of bone morphogenetic protein (BMP-2), bone nodule formation was enhanced to a similar level above that observed with PGE(2) alone in both control and COX-2(-/-) cultures, indicating that BMPs complement COX-2 deficiency and are downstream of prostaglandins. Furthermore, we found that the defect in COX-2(-/-) cultures correlated with significantly reduced levels of cbfa1 and osterix, two genes necessary for bone formation. Addition of PGE(2) rescued this defect, while BMP-2 enhanced cbfa1 and osterix in both COX-2(-/-) and wild-type cultures. Finally, the effects of these agents were additive, indicating that COX-2 is involved in maximal induction of osteogenesis. These results provide a model whereby COX-2 regulates the induction of cbfa1 and osterix to mediate normal skeletal repair.
Figures






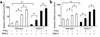

References
-
- Kawaguchi H, et al. Regulation of the two prostaglandin G/H synthases by parathyroid hormone, interleukin-1, cortisol, and prostaglandin E2 in cultured neonatal mouse calvariae. Endocrinology. 1994;135:1157–1164. - PubMed
-
- Raisz LG. Physiologic and pathologic roles of prostaglandins and other eicosanoids in bone metabolism. J Nutr. 1995;125:2024S–2027S. - PubMed
-
- Raisz LG. Prostaglandins and bone: physiology and pathophysiology. Osteoarthritis Cartilage. 1999;7:419–421. - PubMed
-
- Zhang X, et al. Evidence for a direct role of COX-2 in implant wear debris induced osteolysis. J Bone Miner Res. 2001;16:660–669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

