CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse
- PMID: 12045259
- PMCID: PMC150999
- DOI: 10.1172/JCI15078
CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse
Abstract
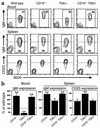
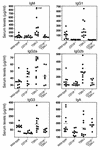
The tight-skin (TSK/+) mouse, a genetic model for human systemic sclerosis (SSc), develops cutaneous fibrosis and autoantibodies against SSc-specific target autoantigens. Although molecular mechanisms explaining the development of fibrosis and autoimmunity in SSc patients or TSK/+ mice remain unknown, we recently demonstrated that SSc patients overexpress CD19, an important regulatory molecule expressed by B lymphocytes. B cells from CD19-deficient mice are hyporesponsive to transmembrane signals, while B cells overexpressing CD19 are hyperresponsive and generate autoantibodies. In this study, TSK/+ B cells also exhibited a hyperresponsive phenotype with decreased surface IgM expression, enhanced serum Ig production, and spontaneous autoantibody production. Moreover, CD19 tyrosine phosphorylation was constitutively augmented in TSK/+ B cells. CD19-mediated [Ca(2+)](i) responses, Vav phosphorylation, and Lyn kinase activity were similarly enhanced. Studies of TSK/+ mice deficient in CD19 expression demonstrated that CD19 deficiency significantly decreased skin fibrosis in TSK/+ mice. Additionally, CD19 loss in TSK/+ mice upregulated surface IgM expression and completely abrogated hyper-gamma-globulinemia and autoantibody production. CD19 deficiency also inhibited IL-6 production by TSK/+ B cells. Thus, chronic B cell activation resulting from augmented CD19 signaling in TSK/+ mice leads to skin sclerosis possibly through IL-6 overproduction as well as autoimmunity.
Figures
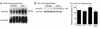
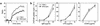


References
-
- Bona C, Rothfield N. Autoantibodies in scleroderma and tightskin mice. Curr Opin Immunol. 1994;6:931–937. - PubMed
-
- Murai C, Saito S, Kasturi KN, Bona CA. Spontaneous occurrence of anti-fibrillin-1 autoantibodies in tight-skin mice. Autoimmunity. 1998;28:151–155. - PubMed
-
- Tan FK, et al. Autoantibodies to the extracellular matrix microfibrillar protein, fibrillin-1, in patients with scleroderma and other connective tissue diseases. J Immunol. 1999;163:1066–1072. - PubMed
-
- Siracusa LD, et al. A tandem duplication within the fibrillin 1 gene is associated with the mouse tight skinmutation. Genome Res. 1996;6:300–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

