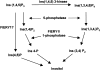
Cell signaling during cold, drought, and salt stress
- PMID: 12045276
- PMCID: PMC151254
- DOI: 10.1105/tpc.000596
Cell signaling during cold, drought, and salt stress
Figures

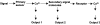



References
-
- Allen, G.J., Chu, S.P., Harrington, C.L., Schumacher, K., Hoffman, T., Tang, Y.Y., Grill, E., and Schroeder, J.I. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411 1053–1057. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

