Phosphorylation of the mitotic regulator Pds1/securin by Cdc28 is required for efficient nuclear localization of Esp1/separase
- PMID: 12050115
- PMCID: PMC186323
- DOI: 10.1101/gad.971402
Phosphorylation of the mitotic regulator Pds1/securin by Cdc28 is required for efficient nuclear localization of Esp1/separase
Abstract
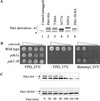
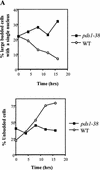
Sister chromatid separation at the metaphase-to-anaphase transition is induced by the proteolytic cleavage of one of the cohesin complex subunits. This process is mediated by a conserved protease called separase. Separase is associated with its inhibitor, securin, until the time of anaphase initiation, when securin is degraded in an anaphase-promoting complex/cyclosome (APC/C)-dependent manner. In budding yeast securin/Pds1 not only inhibits separase/Esp1, but also promotes its nuclear localization. The molecular mechanism and regulation of this nuclear targeting are presently unknown. Here we show that Pds1 is a substrate of the cyclin-dependent kinase Cdc28. Phosphorylation of Pds1 by Cdc28 is important for efficient binding of Pds1 to Esp1 and for promoting the nuclear localization of Esp1. Our results uncover a previously unknown mechanism for regulating the Pds1-Esp1 interaction and shed light on a novel role for Cdc28 in promoting the metaphase-to-anaphase transition in budding yeast.
Figures







References
-
- Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. - PubMed
-
- Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell. 1998;93:1067–1076. - PubMed
-
- Cohen-Fix O. Making and breaking sister chromatid cohesion. Cell. 2001;106:137–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
